Alterations in marginal zone macrophages and marginal zone B cells in old mice
- PMID: 21307289
- PMCID: PMC3420341
- DOI: 10.4049/jimmunol.1001271
Alterations in marginal zone macrophages and marginal zone B cells in old mice
Abstract
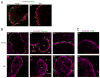
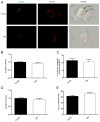
Marginal zones (MZs) are architecturally organized for clearance of and rapid response against blood-borne Ags entering the spleen. MZ macrophages (MZMs) and MZ B cells are particularly important in host defense against T-independent pathogens and may be crucial for the prevention of diseases, such as streptococcal pneumonia, that are devastating in older patients. Our objective was to determine whether there are changes in the cellular components of the MZ between old and young mice. Using immunocytochemistry and a blinded scoring system, we observed gross architectural changes in the MZs of old mice, including reduction in the abundance of MZMs surrounding the MZ sinus as well as disruptions in positioning of mucosal addressin cell adhesion molecule 1 (MAdCAM-1)(+) sinus lining cells and metallophilic macrophages. Loss of frequency of MZMs was corroborated by flow cytometry. A majority of old mice also showed reduced frequency of MZ B cells, which correlated with decreased abundance of MZM in individual old mice. The spleens of old mice showed less deposition of intravenously injected dextran particles within the MZ, likely because of the decreased frequency in MZMs, because SIGN-R1 expression was not reduced on MZM from old mice. The phagocytic ability of individual MZMs was examined using Staphylococcus aureus bioparticles, and no differences in phagocytosis were found between macrophages from young or old spleens. In summary, an anatomical breakdown of the MZ occurs in advanced age, and a reduction in frequency of MZM may affect the ability of the MZM compartment to clear blood-borne Ags and mount proper T-independent immune responses.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures





References
-
- van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. - PubMed
-
- Bondada S, Wu H, Robertson DA, Chelvarajan RL. Accessory cell defect in unresponsiveness of neonates and aged to polysaccharide vaccines. Vaccine. 2000;19:557–565. - PubMed
-
- Butler JC, Shapiro ED, Carlone GM. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107(1A):69S–76S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

