5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids
- PMID: 21307302
- PMCID: PMC3711031
- DOI: 10.1126/scitranslmed.3001571
5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids
Abstract
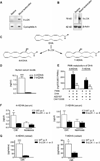
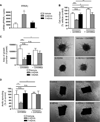
Lipid signaling is dysregulated in many diseases with vascular pathology, including cancer, diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration. We have previously demonstrated that diets enriched in ω-3 polyunsaturated fatty acids (PUFAs) effectively reduce pathological retinal neovascularization in a mouse model of oxygen-induced retinopathy, in part through metabolic products that suppress microglial-derived tumor necrosis factor-α. To better understand the protective effects of ω-3 PUFAs, we examined the relative importance of major lipid metabolic pathways and their products in contributing to this effect. ω-3 PUFA diets were fed to four lines of mice deficient in each key lipid-processing enzyme (cyclooxygenase 1 or 2, or lipoxygenase 5 or 12/15), retinopathy was induced by oxygen exposure; only loss of 5-lipoxygenase (5-LOX) abrogated the protection against retinopathy of dietary ω-3 PUFAs. This protective effect was due to 5-LOX oxidation of the ω-3 PUFA lipid docosahexaenoic acid to 4-hydroxy-docosahexaenoic acid (4-HDHA). 4-HDHA directly inhibited endothelial cell proliferation and sprouting angiogenesis via peroxisome proliferator-activated receptor γ (PPARγ), independent of 4-HDHA's anti-inflammatory effects. Our study suggests that ω-3 PUFAs may be profitably used as an alternative or supplement to current anti-vascular endothelial growth factor (VEGF) treatment for proliferative retinopathy and points to the therapeutic potential of ω-3 PUFAs and metabolites in other diseases of vasoproliferation. It also suggests that cyclooxygenase inhibitors such as aspirin and ibuprofen (but not lipoxygenase inhibitors such as zileuton) might be used without losing the beneficial effect of dietary ω-3 PUFA.
Figures




References
-
- Chen J, Smith L. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. - PubMed
-
- Sapieha P, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S. Proliferative retinopathies: Angiogenesis that blinds. Int. J. Biochem. Cell Biol. 2010;42:5–12. - PubMed
-
- Smith LE. Pathogenesis of retinopathy of prematurity. Semin. Neonatol. 2003;8:469–473. - PubMed
-
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem CN, Jr., Serhan CN, Smith LE. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

