Contributions of skin and muscle afferent input to movement sense in the human hand
- PMID: 21307315
- PMCID: PMC3075285
- DOI: 10.1152/jn.00201.2010
Contributions of skin and muscle afferent input to movement sense in the human hand
Abstract
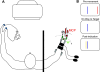
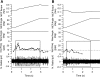
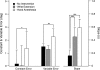
In the stationary hand, static joint-position sense originates from multimodal somatosensory input (e.g., joint, skin, and muscle). In the moving hand, however, it is uncertain how movement sense arises from these different submodalities of proprioceptors. In contrast to static-position sense, movement sense includes multiple parameters such as motion detection, direction, joint angle, and velocity. Because movement sense is both multimodal and multiparametric, it is not known how different movement parameters are represented by different afferent submodalities. In theory, each submodality could redundantly represent all movement parameters, or, alternatively, different afferent submodalities could be tuned to distinctly different movement parameters. The study described in this paper investigated how skin input and muscle input each contributes to movement sense of the hand, in particular, to the movement parameters dynamic position and velocity. Healthy adult subjects were instructed to indicate with the left hand when they sensed the unseen fingers of the right hand being passively flexed at the metacarpophalangeal (MCP) joint through a previously learned target angle. The experimental approach was to suppress input from skin and/or muscle: skin input by anesthetizing the hand, and muscle input by unexpectedly extending the wrist to prevent MCP flexion from stretching the finger extensor muscle. Input from joint afferents was assumed not to play a significant role because the task was carried out with the MCP joints near their neutral positions. We found that, during passive finger movement near the neutral position in healthy adult humans, both skin and muscle receptors contribute to movement sense but qualitatively differently. Whereas skin input contributes to both dynamic position and velocity sense, muscle input may contribute only to velocity sense.
Figures

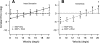

References
-
- Bergenheim M, Ribot-Cisar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res 134: 301–310, 2000 - PubMed
-
- Bevan L, Cordo P, Carlton L, Carlton M. Proprioceptive coordination of movement sequences: discrimination of joint angle versus angular distance. J Neurophysiol 71: 1862–1872, 1994 - PubMed
-
- Brill S, Middleton W, Fisher A. Bier's block: 100 years old and still going strong. Acta Anesthesiol 48: 117–1122, 2004 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

