Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons
- PMID: 21307321
- PMCID: PMC3075294
- DOI: 10.1152/jn.00058.2011
Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons
Abstract
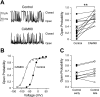
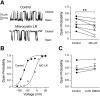
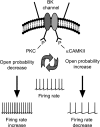
Large conductance K(+) (BK) channels are a key determinant of neuronal excitability. Medial vestibular nucleus (MVN) neurons regulate eye movements to ensure image stabilization during head movement, and changes in their intrinsic excitability may play a critical role in plasticity of the vestibulo-ocular reflex. Plasticity of intrinsic excitability in MVN neurons is mediated by kinases, and BK channels influence excitability, but whether endogenous BK channels are directly modulated by kinases is unknown. Double somatic patch-clamp recordings from MVN neurons revealed large conductance potassium channel openings during spontaneous action potential firing. These channels displayed Ca(2+) and voltage dependence in excised patches, identifying them as BK channels. Recording isolated single channel currents at physiological temperature revealed a novel kinase-mediated bidirectional control in the range of voltages over which BK channels are activated. Application of activated Ca(2+)/calmodulin-dependent kinase II (CAMKII) increased BK channel open probability by shifting the voltage activation range towards more hyperpolarized potentials. An opposite shift in BK channel open probability was revealed by inhibition of phosphatases and was occluded by blockade of protein kinase C (PKC), suggesting that active PKC associated with BK channel complexes in patches was responsible for this effect. Accordingly, direct activation of endogenous PKC by PMA induced a decrease in BK open probability. BK channel activity affects excitability in MVN neurons and bidirectional control of BK channels by CAMKII, and PKC suggests that cellular signaling cascades engaged during plasticity may dynamically control excitability by regulating BK channel open probability.
Figures
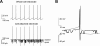

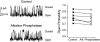

Similar articles
-
Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons.J Neurophysiol. 2002 Apr;87(4):2031-42. doi: 10.1152/jn.00821.2001. J Neurophysiol. 2002. PMID: 11929921
-
Large-conductance voltage- and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C460-70. doi: 10.1152/ajpcell.00325.2013. Epub 2013 Dec 18. Am J Physiol Cell Physiol. 2014. PMID: 24352333 Free PMC article.
-
Characteristics of single large-conductance Ca2+-activated K+ channels and their regulation of action potentials and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus.Am J Physiol Cell Physiol. 2014 Jan 15;306(2):C152-66. doi: 10.1152/ajpcell.00423.2012. Epub 2013 Nov 6. Am J Physiol Cell Physiol. 2014. PMID: 24196530 Free PMC article.
-
Ca(v)1.3 and BK channels for timing and regulating cell firing.Mol Neurobiol. 2010 Dec;42(3):185-98. doi: 10.1007/s12035-010-8151-3. Epub 2010 Nov 20. Mol Neurobiol. 2010. PMID: 21088933 Review.
-
Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity.Prog Neurobiol. 2005 Aug;76(6):349-92. doi: 10.1016/j.pneurobio.2005.10.002. Epub 2005 Nov 2. Prog Neurobiol. 2005. PMID: 16263204 Review.
Cited by
-
Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells.J Neurosci. 2013 Apr 3;33(14):5895-902. doi: 10.1523/JNEUROSCI.4052-12.2013. J Neurosci. 2013. PMID: 23554471 Free PMC article.
-
Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling.Nat Neurosci. 2014 Aug;17(8):1055-63. doi: 10.1038/nn.3744. Epub 2014 Jun 22. Nat Neurosci. 2014. PMID: 24952642 Free PMC article.
-
Characterization of Membrane Patch-Ion Channel Probes for Scanning Ion Conductance Microscopy.Small. 2018 May;14(18):e1702945. doi: 10.1002/smll.201702945. Epub 2017 Dec 11. Small. 2018. PMID: 29226633 Free PMC article.
-
Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes.PLoS One. 2013 Apr 23;8(4):e61844. doi: 10.1371/journal.pone.0061844. Print 2013. PLoS One. 2013. PMID: 23626738 Free PMC article.
-
Vascular CaMKII: heart and brain in your arteries.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C462-78. doi: 10.1152/ajpcell.00341.2015. Epub 2016 Jun 15. Am J Physiol Cell Physiol. 2016. PMID: 27306369 Free PMC article. Review.
References
-
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res 149: 17–31, 2004 - PubMed
-
- Alkon DL, Nelson TJ, Zhao W, Cavallaro S. Time domains of neuronal Ca2+ signaling and associative memory: steps through a calexcitin, ryanodine receptor, K+ channel cascade. Trends Neurosci 21: 529–537, 1998 - PubMed
-
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron 34: 1011–1020, 2002 - PubMed
-
- Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 286: L1275–L1281, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

