Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site-restricted MCMV infection
- PMID: 21307334
- PMCID: PMC3079297
- DOI: 10.1096/fj.10-174656
Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site-restricted MCMV infection
Abstract
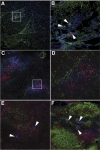
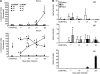
We investigated the hypothesis that salivary gland inoculation stimulates formation of ectopic germinal centers (GCs), transforming the gland into a mucosal inductive site. Intraglandular infection of mice with murine cytomegalovirus (MCMV; control: UV-inactivated MCMV) induces salivary gland ectopic follicles comprising cognate interactions between CD4(+) and B220(+) lymphocytes, IgM(+) and isotype-switched IgG(+) and IgA(+) B cells, antigen presenting cells, and follicular dendritic cells. B cells coexpressed the GC markers GCT (57%) and GL7 (52%), and bound the lectin peanut agglutinin. Lymphoid follicles were characterized by a 2- to 3-fold increase in mRNA for CXCL13 (lymphoid neogenesis), syndecan-1 (plasma cells), Blimp-1 (plasma cell development/differentiation), and a 2- to 6-fold increase for activation-induced cytidine deaminase, PAX5, and the nonexcised rearranged DNA of an IgA class-switch event, supporting somatic hypermutation and class-switch recombination within the salivary follicles. Intraglandular inoculation also provided protection against a systemic MCMV challenge, as evidenced by decreased viral titers (10(5) plaque-forming units to undetectable), and restoration of normal salivary flow rates from a 6-fold decrease. Therefore, these features suggest that the salivary gland participates in oral mucosal immunity via generation of ectopic GCs, which function as ectopic mucosal inductive sites.
Figures



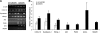
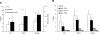
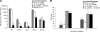

References
-
- Brandtzaeg P. (2007) Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 - PubMed
-
- Takahashi I., Nochi T., Yuki Y., Kiyono H. (2009) New horizon of mucosal immunity and vaccines. Curr. Opin. Immunol. 21, 352–358 - PubMed
-
- Macpherson A. J., McCoy K. D., Johansen F. E., Brandtzaeg P. (2008) The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 - PubMed
-
- Brandtzaeg P., Pabst R. (2004) Let's go mucosal: communication on slippery ground. Trends Immunol. 25, 570–577 - PubMed
-
- Mason K. L., Huffnagle G. B., Noverr M. C., Kao J. Y. (2008) Overview of gut immunology. Adv. Exp. Med. Biol. 635, 1–14 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

