Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases
- PMID: 21307839
- PMCID: PMC3675765
- DOI: 10.1038/ki.2010.516
Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases
Abstract
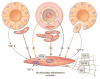
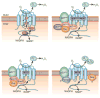
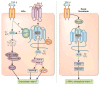
Progression of fibrosis involves interstitial hypercellularity, matrix accumulation, and atrophy of epithelial structures, resulting in loss of normal function and ultimately organ failure. There is common agreement that the fibroblast/myofibroblast is the cell type most responsible for interstitial matrix accumulation and consequent structural deformations associated with fibrosis. During wound healing and progressive fibrotic events, fibroblasts transform into myofibroblasts acquiring smooth muscle features, most notably the expression of alpha-smooth muscle actin and synthesis of mesenchymal cell-related matrix proteins. In renal disease, glomerular mesangial cells also acquire a myofibroblast phenotype and synthesize the same matrix proteins. The origin of interstitial myofibroblasts during fibrosis is a matter of debate, where the cells are proposed to derive from resident fibroblasts, pericytes, perivascular adventitial, epithelial, and/or endothelial sources. Regardless of the origin of the cells, transforming growth factor-beta1 (TGF-β1) is the principal growth factor responsible for myofibroblast differentiation to a profibrotic phenotype and exerts its effects via Smad signaling pathways involving mitogen-activated protein kinase and Akt/protein kinase B. Additionally, reactive oxygen species (ROS) have important roles in progression of fibrosis. ROS are derived from a variety of enzyme sources, of which the nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase family has been identified as a major source of superoxide and hydrogen peroxide generation in the cardiovasculature and kidney during health and disease. Recent evidence indicates that the NAD(P)H oxidase homolog Nox4 is most accountable for ROS-induced fibroblast and mesangial cell activation, where it has an essential role in TGF-β1 signaling of fibroblast activation and differentiation into a profibrotic myofibroblast phenotype and matrix production. Information on the role of ROS in mesangial cell and fibroblast signaling is incomplete, and further research on myofibroblast differentiation during fibrosis is warranted.
Conflict of interest statement
The authors declared no competing interests.
Figures
Similar articles
-
NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts.J Am Soc Nephrol. 2010 Jan;21(1):93-102. doi: 10.1681/ASN.2009020146. Epub 2009 Nov 19. J Am Soc Nephrol. 2010. PMID: 19926889 Free PMC article.
-
RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species.Am J Physiol Renal Physiol. 2014 Jul 15;307(2):F159-71. doi: 10.1152/ajprenal.00546.2013. Epub 2014 May 28. Am J Physiol Renal Physiol. 2014. PMID: 24872317 Free PMC article.
-
NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts.Circ Res. 2005 Oct 28;97(9):900-7. doi: 10.1161/01.RES.0000187457.24338.3D. Epub 2005 Sep 22. Circ Res. 2005. PMID: 16179589
-
NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses.Redox Biol. 2014 Jan 20;2:267-72. doi: 10.1016/j.redox.2014.01.012. eCollection 2014. Redox Biol. 2014. PMID: 24494202 Free PMC article. Review.
-
Therapeutic targeting of redox signaling in myofibroblast differentiation and age-related fibrotic disease.Oxid Med Cell Longev. 2012;2012:458276. doi: 10.1155/2012/458276. Epub 2012 Oct 22. Oxid Med Cell Longev. 2012. PMID: 23150749 Free PMC article. Review.
Cited by
-
Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling.J Biol Chem. 2013 Jan 11;288(2):770-7. doi: 10.1074/jbc.M112.431973. Epub 2012 Nov 30. J Biol Chem. 2013. PMID: 23204521 Free PMC article.
-
Bilirubin attenuates the renal tubular injury by inhibition of oxidative stress and apoptosis.BMC Nephrol. 2013 May 17;14:105. doi: 10.1186/1471-2369-14-105. BMC Nephrol. 2013. PMID: 23683031 Free PMC article.
-
Hydroxysafflor Yellow A Ameliorates Renal Fibrosis by Suppressing TGF-β1-Induced Epithelial-to-Mesenchymal Transition.PLoS One. 2016 Apr 18;11(4):e0153409. doi: 10.1371/journal.pone.0153409. eCollection 2016. PLoS One. 2016. PMID: 27088510 Free PMC article.
-
Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells.PLoS One. 2013 Apr 12;8(4):e60471. doi: 10.1371/journal.pone.0060471. Print 2013. PLoS One. 2013. PMID: 23593224 Free PMC article.
-
Role of NADPH Oxidase in Metabolic Disease-Related Renal Injury: An Update.Oxid Med Cell Longev. 2016;2016:7813072. doi: 10.1155/2016/7813072. Epub 2016 Aug 15. Oxid Med Cell Longev. 2016. PMID: 27597884 Free PMC article. Review.
References
-
- Strutz F, Muller GA. Mechanisms of renal fibrogenesis. In: Neilson EG, Couser WG, editors. Immunologic renal diseases. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 73–101.
-
- Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol - Renal Physiol. 2009;296:F1239–F1244. - PubMed
-
- Hughson MD. End-stage renal disease. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6. Philadelphia: Lippincott-Raven Publishers; 2007. pp. 1307–1346.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

