Prospects for development of a vaccine to prevent and control vaginal candidiasis
- PMID: 21308461
- PMCID: PMC3062364
- DOI: 10.1007/s11908-010-0143-y
Prospects for development of a vaccine to prevent and control vaginal candidiasis
Abstract
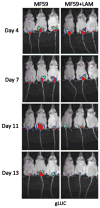
A vaccine against recurrent vulvovaginal candidiasis (RVVC) would benefit a large number of women who suffer from this debilitating syndrome. To date, several antigen formulations have been tested with modest results. In this article, we review the latest vaccine study reported in the literature. The candidate is a β-glucan conjugate administered with a human compatible adjuvant. Results in a mouse model of vaginitis were again modest for protection. However, the study included live animal imaging to quantify fungal burden; animals were challenged with a Candida strain carrying a gene encoding a glycophosphatidylinositol (GPI)-linked cell wall protein and luciferase. Fungal burden was expressed as photons following substrate administration. Protection appeared to be mediated by β-glucan antibodies. Although modest protection was observed, the imaging system was less variable than semi-quantitative plate counts of vaginal lavage fluid. Despite these advances in evaluating protection, a vaccine candidate against RVVC worthy of clinical testing remains elusive.
Conflict of interest statement
Figures
Similar articles
-
A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique.Vaccine. 2010 Feb 17;28(7):1717-25. doi: 10.1016/j.vaccine.2009.12.021. Epub 2009 Dec 25. Vaccine. 2010. PMID: 20038431
-
Recurrent vulvovaginal candidiasis.Am J Obstet Gynecol. 2016 Jan;214(1):15-21. doi: 10.1016/j.ajog.2015.06.067. Epub 2015 Jul 9. Am J Obstet Gynecol. 2016. PMID: 26164695 Review.
-
Analysis of pathogenic factors of Candida albicans and the effect of vaginal immunization on recurrent vulvovaginal candidiasis in mice.J Obstet Gynaecol Res. 2022 Mar;48(3):857-865. doi: 10.1111/jog.15140. Epub 2021 Dec 30. J Obstet Gynaecol Res. 2022. PMID: 34970814
-
Patients with recurrent vulvovaginal candidiasis exhibit a decrease in both the fungicidal activity of neutrophils and the proliferation of peripheral blood mononuclear cells.Mycoses. 2024 Apr;67(4):e13720. doi: 10.1111/myc.13720. Mycoses. 2024. PMID: 38551114
-
Vulvovaginal Candida albicans infections: pathogenesis, immunity and vaccine prospects.BJOG. 2015 May;122(6):785-794. doi: 10.1111/1471-0528.12994. Epub 2014 Jul 23. BJOG. 2015. PMID: 25052208 Review.
Cited by
-
Protective efficacy of the pan-fungal vaccine NXT-2 against vulvovaginal candidiasis in a murine model.NPJ Vaccines. 2025 Jun 2;10(1):112. doi: 10.1038/s41541-025-01171-4. NPJ Vaccines. 2025. PMID: 40456775 Free PMC article.
-
Recent progress in vaccines against fungal diseases.Curr Opin Microbiol. 2012 Aug;15(4):427-33. doi: 10.1016/j.mib.2012.04.004. Epub 2012 May 6. Curr Opin Microbiol. 2012. PMID: 22564747 Free PMC article. Review.
-
Innate Inspiration: Antifungal Peptides and Other Immunotherapeutics From the Host Immune Response.Front Immunol. 2020 Sep 17;11:2177. doi: 10.3389/fimmu.2020.02177. eCollection 2020. Front Immunol. 2020. PMID: 33072081 Free PMC article. Review.
-
Fungal cell wall vaccines: an update.J Med Microbiol. 2012 Jul;61(Pt 7):895-903. doi: 10.1099/jmm.0.041665-0. Epub 2012 Jan 19. J Med Microbiol. 2012. PMID: 22267544 Free PMC article. Review.
-
New approaches in the development of a vaccine for mucosal candidiasis: progress and challenges.Front Microbiol. 2012 Aug 13;3:294. doi: 10.3389/fmicb.2012.00294. eCollection 2012. Front Microbiol. 2012. PMID: 22905033 Free PMC article.
References
-
- Sobel JD, Faro S, Force R, et al. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. - PubMed
-
- Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. - PubMed
-
- Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

