Prospects for development of a vaccine to prevent and control vaginal candidiasis
- PMID: 21308461
- PMCID: PMC3062364
- DOI: 10.1007/s11908-010-0143-y
Prospects for development of a vaccine to prevent and control vaginal candidiasis
Abstract
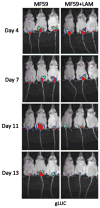
A vaccine against recurrent vulvovaginal candidiasis (RVVC) would benefit a large number of women who suffer from this debilitating syndrome. To date, several antigen formulations have been tested with modest results. In this article, we review the latest vaccine study reported in the literature. The candidate is a β-glucan conjugate administered with a human compatible adjuvant. Results in a mouse model of vaginitis were again modest for protection. However, the study included live animal imaging to quantify fungal burden; animals were challenged with a Candida strain carrying a gene encoding a glycophosphatidylinositol (GPI)-linked cell wall protein and luciferase. Fungal burden was expressed as photons following substrate administration. Protection appeared to be mediated by β-glucan antibodies. Although modest protection was observed, the imaging system was less variable than semi-quantitative plate counts of vaginal lavage fluid. Despite these advances in evaluating protection, a vaccine candidate against RVVC worthy of clinical testing remains elusive.
Conflict of interest statement
Figures
References
-
- Sobel JD, Faro S, Force R, et al. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. - PubMed
-
- Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. - PubMed
-
- Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

