Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33
- PMID: 21308681
- PMCID: PMC3085255
- DOI: 10.1002/eji.201040718
Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33
Abstract
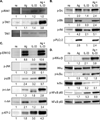
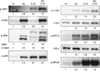
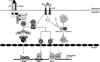
IL-33 is associated with atopic and autoimmune diseases and, as reported here, it interacts synergistically with Ag to markedly enhance production of inflammatory cytokines in rodent mast cells even in the absence of degranulation. Investigation of the underlying mechanisms revealed that synergy in signaling occurred at the level of TGF-β-activated kinase 1, which was then transmitted downstream through JNK, p38 MAP kinase, and AP-1. Stimulation of the Ca(2+) /calcineurin/NFAT pathway by Ag, which IL-33 did not, was critical for the synergy between Ag and IL-33. For example, selective stimulation of the NFAT pathway by thapsigargin also markedly enhanced responses to IL-33 in a calcineurin-dependent manner. As indicated by luciferase-reporter assays, IL-33 failed to stimulate the transcriptional activities of NFAT and AP-1 but augmented the activation of these transcription factors by Ag or thapsigargin. Robust stimulation of NF-κB transcriptional activity by IL-33 was also essential for the synergy. These and pharmacologic data suggested that the enhanced production of cytokines resulted in part from amplification of the activation of AP-1 and NFAT as well as co-operative interactions among transcription factors. IL-33 may retune mast cell responses to Ag toward enhanced cytokine production and thus determine the symptoms and severity of Ag-dependent allergic and autoimmune diseases.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: Recent Advances. Annu.Rev.Immunol. 2005;23:749–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

