Role of C/EBP-β, p38 MAPK, and MKK6 in IL-1β-mediated C3 gene regulation in astrocytes
- PMID: 21308746
- PMCID: PMC3072290
- DOI: 10.1002/jcb.23032
Role of C/EBP-β, p38 MAPK, and MKK6 in IL-1β-mediated C3 gene regulation in astrocytes
Abstract
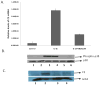
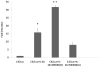
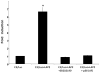
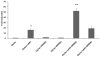
Complement component C3, the central player in the complement cascade and the pro-inflammatory cytokine IL-1β is expressed by activated glial cells and may contribute to neurodegeneration. This study examines the regulation of the expression of C3 by IL-1β in astroglial cells focusing on the role of the upstream kinase MKK6, p38-α MAPK, and C/EBP-β isoforms (LAP1, LAP2, or LIP) in astroglial cells. Activation of human astroglial cell line, U373 with IL-1β, led to the induction of C3 mRNA and protein expression as determined by real-time RT-PCR and Western blot analysis, respectively. This induction was suppressed by the pharmacological inhibitor of p38 MAPK (i.e., SB202190-HCl), suggesting the involvement of p38 MAPK in C3 gene expression. IL-1β also induced C3 promoter activity in U373 cells in a MAP kinase- and C/EBP-β-dependent manner. Cotransfection of C3 luciferase reporter construct with constitutively active form of the upstream kinase in the MAP kinase cascade, that is, MKK6 (the immediate upstream activator of p38 kinase) resulted in marked stimulation of the promoter activity, whereas overexpression of a dominant negative forms of MKK6 and p38α MAPK inhibited C3 promoter activity. Furthermore, a mutant form of C/EBP-β, LAP(T235A) showed reduction in IL-1β-mediated C3 promoter activation. These results suggest that the p38α, MAPK, and MKK6 play prominent roles in IL-1β and C/EBP-β-mediated C3 gene expression in astrocytes.
Copyright © 2011 Wiley-Liss, Inc.
Figures




Similar articles
-
Regulation of complement component C3 in astrocytes by IL-1beta and morphine.J Neuroimmune Pharmacol. 2008 Mar;3(1):43-51. doi: 10.1007/s11481-007-9096-9. Epub 2007 Nov 14. J Neuroimmune Pharmacol. 2008. PMID: 18247123
-
Interleukin-1beta regulation of the human brain natriuretic peptide promoter involves Ras-, Rac-, and p38 kinase-dependent pathways in cardiac myocytes.Hypertension. 1999 Jan;33(1 Pt 2):283-9. doi: 10.1161/01.hyp.33.1.283. Hypertension. 1999. PMID: 9931118
-
IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta , NF-kappaB, and AP-1 activation.Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3356-65. doi: 10.1152/ajpheart.00928.2007. Epub 2007 Oct 5. Am J Physiol Heart Circ Physiol. 2007. PMID: 17921324
-
MAP kinase kinase 6-p38 MAP kinase signaling cascade regulates cyclooxygenase-2 expression in cardiac myocytes in vitro and in vivo.Circ Res. 2003 Apr 18;92(7):757-64. doi: 10.1161/01.RES.0000067929.01404.03. Epub 2003 Mar 20. Circ Res. 2003. PMID: 12649265
-
NF-kappaB- and C/EBPbeta-driven interleukin-1beta gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1beta release from Helicobacter pylori lipopolysaccharide-stimulated macrophages.J Biol Chem. 2005 Feb 11;280(6):4279-88. doi: 10.1074/jbc.M412820200. Epub 2004 Nov 23. J Biol Chem. 2005. PMID: 15561713
Cited by
-
Downregulation of HMGB1 carried by macrophage-derived extracellular vesicles delays atherosclerotic plaque formation through Caspase-11-dependent macrophage pyroptosis.Mol Med. 2024 Mar 16;30(1):38. doi: 10.1186/s10020-023-00753-z. Mol Med. 2024. PMID: 38493291 Free PMC article.
-
High glucose-induced complement component 3 up-regulation via RAGE-p38MAPK-NF-κB signalling in astrocytes: In vivo and in vitro studies.J Cell Mol Med. 2018 Dec;22(12):6087-6098. doi: 10.1111/jcmm.13884. Epub 2018 Sep 24. J Cell Mol Med. 2018. PMID: 30246940 Free PMC article.
-
Nonstructural Protein 1 of Variant PEDV Plays a Key Role in Escaping Replication Restriction by Complement C3.J Virol. 2022 Sep 28;96(18):e0102422. doi: 10.1128/jvi.01024-22. Epub 2022 Aug 29. J Virol. 2022. PMID: 36037478 Free PMC article.
-
Hypersensitivity Responses in the Central Nervous System.Front Immunol. 2015 Oct 7;6:517. doi: 10.3389/fimmu.2015.00517. eCollection 2015. Front Immunol. 2015. PMID: 26500654 Free PMC article. Review.
-
Non-Metabolic Role of PKM2 in Regulation of the HIV-1 LTR.J Cell Physiol. 2017 Mar;232(3):517-525. doi: 10.1002/jcp.25445. Epub 2016 Jun 10. J Cell Physiol. 2017. PMID: 27249540 Free PMC article.
References
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
-
- Andoh A, Fujiyama Y, Shimada M, Bamba T. Modulation of complement component (C3 and factor B) biosynthesis by a histone deacetylase inhibitor in human intestinal epithelial cells. Int J Mol Med. 2000;6:51–54. - PubMed
-
- Aouadi M, Jager J, Laurent K, Gonzalez T, Cormont M, Binétruy B, Le Marchand-Brustel Y, Tanti JF, Bost F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007;581:5591–5596. - PubMed
-
- Barnum SR, Jones JL, Benveniste EN. Interferon-gamma regulation of C3 gene expression in human astroglioma cells. J Neuroimmunol. 1992;38:275–282. - PubMed
-
- Barnum SR, Jones JL, Benveniste EN. Interleukin-1 and tumor necrosis factor-mediated regulation of C3 gene expression in human astroglioma cells. Glia. 1993;7:225–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

