Skeletal recovery after weaning does not require PTHrP
- PMID: 21308774
- PMCID: PMC3179289
- DOI: 10.1002/jbmr.339
Skeletal recovery after weaning does not require PTHrP
Abstract
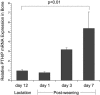
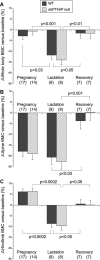
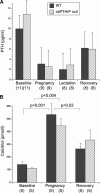
Mice lose 20% to 25% of trabecular bone mineral content (BMC) during lactation and restore it after weaning through unknown mechanisms. We found that tibial Pthrp mRNA expression was upregulated fivefold by 7 days after weaning versus end of lactation in wild-type (WT) mice. To determine whether parathyroid hormone-related protein (PTHrP) stimulates bone formation after weaning, we studied a conditional knockout in which PTHrP is deleted from preosteoblasts and osteoblasts by collagen I promoter-driven Cre (Cre(ColI) ). These mice are osteopenic as adults but have normal serum calcium, calcitriol, and parathyroid hormone (PTH). Pairs of Pthrp(flox/flox) ;Cre(ColI) (null) and WT;Cre(ColI) (WT) females were mated and studied through pregnancy, lactation, and 3 weeks of postweaning recovery. By end of lactation, both genotypes lost lumbar spine BMC: WT declined by 20.6% ± 3.3%, and null decreased by 22.5% ± 3.5% (p < .0001 versus baseline; p = NS between genotypes). During postweaning recovery, both restored BMC to baseline: WT to -3.6% ± 3.7% and null to 0.3% ± 3.7% (p = NS versus baseline or between genotypes). Similar loss and full recovery of BMC were seen at the whole body and hind limb. Histomorphometry confirmed that nulls had lower bone mass at baseline and that this was equal to the value achieved after weaning. Osteocalcin, propeptide of type 1 collagen (P1NP), and deoxypyridinoline increased equally during recovery in WT and null mice; PTH decreased and calcitriol increased equally; serum calcium was unchanged. Urine calcium increased during recovery but remained no different between genotypes. Although osteoblast-derived PTHrP is required to maintain adult bone mass and Pthrp mRNA upregulates in bone after weaning, it is not required for recovery of bone mass after lactation. The factors that stimulate postweaning bone formation remain unknown.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures


Similar articles
-
Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone.J Bone Miner Res. 2013 Sep;28(9):1987-2000. doi: 10.1002/jbmr.1925. J Bone Miner Res. 2013. PMID: 23505097
-
Absence of Calcitriol Causes Increased Lactational Bone Loss and Lower Milk Calcium but Does Not Impair Post-lactation Bone Recovery in Cyp27b1 Null Mice.J Bone Miner Res. 2018 Jan;33(1):16-26. doi: 10.1002/jbmr.3217. Epub 2017 Aug 2. J Bone Miner Res. 2018. PMID: 28686309
-
Maternal loss of 24-hydroxylase causes increased intestinal calcium absorption and hypercalcemia during pregnancy but reduced skeletal resorption during lactation in mice.J Bone Miner Res. 2024 Nov 29;39(12):1793-1808. doi: 10.1093/jbmr/zjae166. J Bone Miner Res. 2024. PMID: 39385466 Free PMC article.
-
[Parathyroid hormone related peptide (PTHrP) and bone metabolism].Arch Physiol Biochem. 1995 Apr;103(1):3-13. doi: 10.3109/13813459509007556. Arch Physiol Biochem. 1995. PMID: 8574773 Review. French.
-
Bone metabolism during pregnancy.Ann Endocrinol (Paris). 2016 Jun;77(2):163-8. doi: 10.1016/j.ando.2016.04.004. Epub 2016 May 2. Ann Endocrinol (Paris). 2016. PMID: 27157104 Review.
Cited by
-
Parathyroid hormone-related protein overexpression protects goat mammary gland epithelial cells from calcium-sensing receptor activation-induced apoptosis.Mol Biol Rep. 2015 Jan;42(1):233-43. doi: 10.1007/s11033-014-3763-8. Epub 2014 Sep 30. Mol Biol Rep. 2015. PMID: 25266236
-
Loss of 24-hydroxylated catabolism increases calcitriol and fibroblast growth factor 23 and alters calcium and phosphate metabolism in fetal mice.JBMR Plus. 2024 Jan 29;8(5):ziae012. doi: 10.1093/jbmrpl/ziae012. eCollection 2024 May. JBMR Plus. 2024. PMID: 38577520 Free PMC article.
-
Incorporation of Flaxseed Flour as a Dietary Source for ALA Increases Bone Density and Strength in Post-Partum Female Rats.Lipids. 2017 Apr;52(4):327-333. doi: 10.1007/s11745-017-4245-2. Epub 2017 Mar 21. Lipids. 2017. PMID: 28324248
-
Memory T-Cells Contribute to Calcium Release from Bones during Lactation in Mice.Nutrients. 2024 Sep 28;16(19):3289. doi: 10.3390/nu16193289. Nutrients. 2024. PMID: 39408256 Free PMC article.
-
Morphological, hormonal, and molecular changes in different maternal tissues during lactation and post-lactation.J Physiol Sci. 2019 Nov;69(6):825-835. doi: 10.1007/s12576-019-00714-4. Epub 2019 Sep 28. J Physiol Sci. 2019. PMID: 31564033 Free PMC article. Review.
References
-
- Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium and lactation. Endocr Rev. 1997;18:832–872. - PubMed
-
- Kovacs CS, El-Hajj Fuleihan G. Calcium and bone disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2006;35:21–51. - PubMed
-
- Wysolmerski JJ. Conversations between breast and bone: physiological bone loss during lactation as evolutionary template for osteolysis in breast cancer and pathological bone loss after menopause. BoneKEy. 2007;4:209–225.
-
- Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab. 2001;86:2344–2348. - PubMed
-
- Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

