A secretory system for bacterial production of high-profile protein targets
- PMID: 21308845
- PMCID: PMC3064838
- DOI: 10.1002/pro.593
A secretory system for bacterial production of high-profile protein targets
Abstract
Escherichia coli represents a robust, inexpensive expression host for the production of recombinant proteins. However, one major limitation is that certain protein classes do not express well in a biologically relevant form using standard expression approaches in the cytoplasm of E. coli. To improve the usefulness of the E. coli expression platform we have investigated combinations of promoters and selected N-terminal fusion tags for the extracellular expression of human target proteins. A comparative study was conducted on 24 target proteins fused to outer membrane protein A (OmpA), outer membrane protein F (OmpF) and osmotically inducible protein Y (OsmY). Based on the results of this initial study, we carried out an extended expression screen employing the OsmY fusion and multiple constructs of a more diverse set of human proteins. Using this high-throughput compatible system, we clearly demonstrate that secreted biomedically relevant human proteins can be efficiently retrieved and purified from the growth medium.
Copyright © 2011 The Protein Society.
Figures


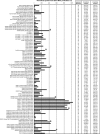


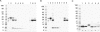
Similar articles
-
Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system.PLoS One. 2014 May 13;9(5):e97367. doi: 10.1371/journal.pone.0097367. eCollection 2014. PLoS One. 2014. PMID: 24824752 Free PMC article.
-
Random and combinatorial mutagenesis for improved total production of secretory target protein in Escherichia coli.Sci Rep. 2021 Mar 5;11(1):5290. doi: 10.1038/s41598-021-84859-6. Sci Rep. 2021. PMID: 33674702 Free PMC article.
-
The rate of folding dictates substrate secretion by the Escherichia coli hemolysin type 1 secretion system.J Biol Chem. 2010 Dec 24;285(52):40573-80. doi: 10.1074/jbc.M110.173658. Epub 2010 Oct 22. J Biol Chem. 2010. PMID: 20971850 Free PMC article.
-
An engineered autotransporter-based surface expression vector enables efficient display of Affibody molecules on OmpT-negative E. coli as well as protease-mediated secretion in OmpT-positive strains.Microb Cell Fact. 2014 Dec 30;13:179. doi: 10.1186/s12934-014-0179-z. Microb Cell Fact. 2014. PMID: 25547008 Free PMC article.
-
ABC transporters involved in the biogenesis of the outer membrane in gram-negative bacteria.Biosci Biotechnol Biochem. 2011;75(6):1044-54. doi: 10.1271/bbb.110115. Epub 2011 Jun 13. Biosci Biotechnol Biochem. 2011. PMID: 21670534 Review.
Cited by
-
Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system.PLoS One. 2014 May 13;9(5):e97367. doi: 10.1371/journal.pone.0097367. eCollection 2014. PLoS One. 2014. PMID: 24824752 Free PMC article.
-
Accurate de novo design of hyperstable constrained peptides.Nature. 2016 Oct 20;538(7625):329-335. doi: 10.1038/nature19791. Epub 2016 Sep 14. Nature. 2016. PMID: 27626386 Free PMC article.
-
Extracellular production of Ulp1403-621 in leaky E. coli and its application in antimicrobial peptide production.Appl Microbiol Biotechnol. 2022 Dec;106(23):7805-7817. doi: 10.1007/s00253-022-12235-z. Epub 2022 Oct 19. Appl Microbiol Biotechnol. 2022. PMID: 36260100
-
Mechanisms Involved in the Active Secretion of CTX-M-15 β-Lactamase by Pathogenic Escherichia coli ST131.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0066321. doi: 10.1128/AAC.00663-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310213 Free PMC article.
-
A High Throughput Assay for Measuring Secreted Protein Based on a de novo Fluorescent Reporter Reveals Regulatory and Structural Insights in Salmonella Type Three Secretion System.bioRxiv [Preprint]. 2025 Jan 18:2025.01.17.633628. doi: 10.1101/2025.01.17.633628. bioRxiv. 2025. Update in: Protein Sci. 2025 Jul;34(7):e70183. doi: 10.1002/pro.70183. PMID: 39868124 Free PMC article. Updated. Preprint.
References
-
- Berndt C, Lillig CH, Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta. 2008;1783:641–650. - PubMed
-
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22:1399–1408. - PubMed
-
- Lazdunski CJ. Pore-forming colicins: synthesis, extracellular release, mode of action, immunity. Biochimie. 1988;70:1291–1296. - PubMed
-
- Filloux A, Voulhoux R, Ize B, Gerard F, Ball G, Wu LF. Use of colicin-based genetic tools for studying bacterial protein transport. Biochimie. 2002;84:489–497. - PubMed
-
- Hughes C, Stanley P, Koronakis V. E. coli hemolysin interactions with prokaryotic and eukaryotic cell membranes. Bioessays. 1992;14:519–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

