α,β-Unsaturated acyl azoliums from N-heterocyclic carbene catalyzed reactions: observation and mechanistic investigation
- PMID: 21308930
- PMCID: PMC3189509
- DOI: 10.1002/anie.201005352
α,β-Unsaturated acyl azoliums from N-heterocyclic carbene catalyzed reactions: observation and mechanistic investigation
Abstract
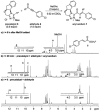
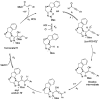
Catalytically generated acyl azoliums I and their α,β-unsaturated counterparts II are thought to be key reactive intermediates in a rapidly growing number of transformations promoted by N-heterocyclic carbene (NHC) catalysts.[1] Acyl azoliums are invoked in the postulated catalytic cycles of nearly all of the new NHC-catalyzed reactions of α-functionalized aldehydes reported since 2004, in which they are generally assumed to possess the reactivity of an activated carboxylic acid, that is, analogous to an activated ester.[2] In NHC-catalyzed processes, they are most often obtained through internal redox reactions of functionalized aldehydes but have also been prepared by oxidations of the Breslow intermediates[3] or additions to ketenes.[4] Acyl azoliums I are important intermediates in thiamine pyrophosphate (ThPP) dependent enzymatic reactions.[5] Townsend et al. have recently proposed that unsaturated acyl azolium III is the key intermediate in clavulanic acid biosynthesis;[6] despite careful efforts, III or its analogues II have never been characterized or independently synthesized. Here, we document the observation and characterization of α,β-unsaturated acyl azoliums 1 and 2 (Scheme 1) and demonstrate that their corresponding hemiacetals (1′ and 2”) are the kinetically important intermediates in both their acylation and annulation reactions.
Figures





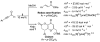

References
-
- Moore JL, Rovis T. In: Topics in Current Chemistry. List B, editor. Vol. 291. Springer; Berlin: 2009. pp. 77–144. - PMC - PubMed
- Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. - PubMed
- Zeitler K. Angew Chem. 2005;117:7674–7678.
- Angew Chem Int Ed. 2005;44:7506–7510. - PubMed
- Enders D, Balensiefer T. Acc Chem Res. 2004;37:534–541. - PubMed
- Chiang PC, Bode JW. In: N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools. Díez-González S, editor. Royal Society of Chemistry; Cambridge: 2010. pp. 399–435.
-
- Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. - PubMed
- Reynolds NT, Read de Alaniz J, Rovis T. J Am Chem Soc. 2004;126:9518–9519. - PubMed
- Chow KYK, Bode JW. J Am Chem Soc. 2004;126:8126–8127. - PubMed
- Burstein C, Glorius F. Angew Chem. 2004;116:6331–6334. - PubMed
- Angew Chem Int Ed. 2004;43:6205–6208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

