Dendritic cells from humans with hypomorphic mutations in IKBKG/NEMO have impaired mitogen-activated protein kinase activity
- PMID: 21309033
- PMCID: PMC3134100
- DOI: 10.1002/humu.21439
Dendritic cells from humans with hypomorphic mutations in IKBKG/NEMO have impaired mitogen-activated protein kinase activity
Abstract
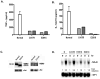
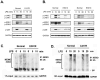
The covalent attachment of lysine 63-linked polyubiquitin to the zinc-finger domain of IKBKG/NEMO (also known as IKKγ) is necessary for full activation of NF-κB. Impairments of this biochemical mechanism explain the deleterious effects of hypomorphic NEMO mutations on NF-κB signaling function in humans suffering from X-linked ectodermal dysplasia and immunodeficiency. Nevertheless, the biological function of the NEMO zinc-finger domain in the regulation of mitogen-activated protein kinase (MAPK) activity is poorly understood. Here we show that dendritic cells from patients with EDI caused by a C-terminal E391X deletion of the zinc finger of NEMO exhibit impaired MAPK activation in response to lipopolysaccharide (LPS) stimulation. Interestingly, DCs from patients with a C417R missense mutation within the zinc finger domain of NEMO in which ubiquitination of NEMO is preserved are also defective in JNK and ERK activity following LPS stimulation. Our findings indicate that the structural integrity of the NEMO ZF domain is more important than its polyubiquitination for full activation of the MAPK. Furthermore, phosphorylation and polyubiquitination of upstream TAK1 were significantly reduced in the E391X zinc-finger deleted patients, indicating that the NEMO zinc finger may play an important role in assembling the proximal signaling complex for MAPK activation.
This article is a US Government work, and, as such, is in the public domain in the United States of America. Published 2011 by Wiley-Liss, Inc.
Conflict of interest statement
Figures

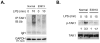
References
-
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424(6950):797–801. - PubMed
-
- Cordier F, Vinolo E, Veron M, Delepierre M, Agou F. Solution structure of NEMO zinc finger and impact of an anhidrotic ectodermal dysplasia with immunodeficiency-related point mutation. J Mol Biol. 2008;377(5):1419–32. - PubMed
-
- Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–85. - PubMed
-
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–83. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

