New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations
- PMID: 21309044
- PMCID: PMC3383654
- DOI: 10.1002/humu.21462
New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations
Abstract
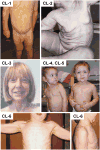
Autosomal dominant cutis laxa (ADCL) is characterized by a typical facial appearance and generalized loose skin folds, occasionally associated with aortic root dilatation and emphysema. We sequenced exons 28-34 of the ELN gene in five probands with ADCL features and found five de novo heterozygous mutations: c.2296_2299dupGCAG (CL-1), c.2333delC (CL-2), c.2137delG (CL-3), c.2262delA (monozygotic twin CL-4 and CL-5), and c.2124del25 (CL-6). Four probands (CL-1,-2,-3,-6) presented with progressive aortic root dilatation. CL-2 and CL-3 also had bicuspid aortic valves. CL-2 presented with severe emphysema. Electron microscopy revealed elastic fiber fragmentation and diminished dermal elastin deposition. RT-PCR studies showed stable mutant mRNA in all patients. Exon 32 skipping explains a milder phenotype in patients with exon 32 mutations. Mutant protein expression in fibroblast cultures impaired deposition of tropoelastin onto microfibril-containing fibers, and enhanced tropoelastin coacervation and globule formation leading to lower amounts of mature, insoluble elastin. Mutation-specific effects also included endoplasmic reticulum stress and increased apoptosis. Increased pSMAD2 staining in ADCL fibroblasts indicated enhanced transforming growth factor beta (TGF-β) signaling. We conclude that ADCL is a systemic disease with cardiovascular and pulmonary complications, associated with increased TGF-β signaling and mutation-specific differences in endoplasmic reticulum stress and apoptosis.
© 2011 Wiley-Liss, Inc.
Figures






References
-
- Basel-Vanagaite L, Sarig O, Hershkovitz D, Fuchs-Telem D, Rapaport D, Gat A, Isman G, Shirazi I, Shohat M, Enk CD, Birk E, Kohlhase J, Matysiak-Scholze U, Maya I, Knopf C, Peffekoven A, Hennies HC, Bergman R, Horowitz M, Ishida-Yamamoto A, Sprecher E. RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am J Hum Genet. 2009;85:254–263. - PMC - PubMed
-
- Bicknell LS, Pitt J, Aftimos S, Ramadas R, Maw MA, Robertson SP. A missense mutation in ALDH18A1, encoding Delta1-pyrroline-5-carboxylate synthase (P5CS), causes an autosomal recessive neurocutaneous syndrome. Eur J Hum Genet. 2008;16:1176–1186. - PubMed
-
- Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem. 2005;280(49):40939–40947. - PubMed
-
- Brown FR, 3rd, Holbrook KA, Byers PH, Stewart D, Dean J, Pyeritz RE. Cutis laxa. Johns Hopkins Med J. 1982;150:148–153. - PubMed
-
- Brown-Augsburger P, Broekelmann T, Mecham L, Mercer R, Gibson MA, Cleary EG, Abrams WR, Rosenbloom J, Mecham RP. Microfibril-associated glycoprotein binds to the carboxyl-terminal domain of tropoelastin and is a substrate for transglutaminase. J Biol Chem. 1994;269:28443–28449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

