Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates
- PMID: 21310050
- PMCID: PMC3042927
- DOI: 10.1186/1754-6834-4-3
Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates
Abstract
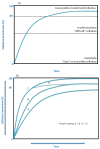
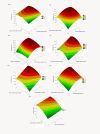
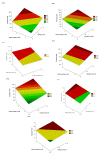
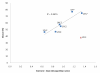
A range of lignocellulosic feedstocks (including agricultural, softwood and hardwood substrates) were pretreated with either sulfur dioxide-catalyzed steam or an ethanol organosolv procedure to try to establish a reliable assessment of the factors governing the minimum protein loading that could be used to achieve efficient hydrolysis. A statistical design approach was first used to define what might constitute the minimum protein loading (cellulases and β-glucosidase) that could be used to achieve efficient saccharification (defined as at least 70% glucan conversion) of the pretreated substrates after 72 hours of hydrolysis. The likely substrate factors that limit cellulose availability/accessibility were assessed, and then compared with the optimized minimum amounts of protein used to obtain effective hydrolysis. The optimized minimum protein loadings to achieve efficient hydrolysis of seven pretreated substrates ranged between 18 and 63 mg protein per gram of glucan. Within the similarly pretreated group of lignocellulosic feedstocks, the agricultural residues (corn stover and corn fiber) required significantly lower protein loadings to achieve efficient hydrolysis than did the pretreated woody biomass (poplar, douglas fir and lodgepole pine). Regardless of the substantial differences in the source, structure and chemical composition of the feedstocks, and the difference in the pretreatment technology used, the protein loading required to achieve efficient hydrolysis of lignocellulosic substrates was strongly dependent on the accessibility of the cellulosic component of each of the substrates. We found that cellulose-rich substrates with highly accessible cellulose, as assessed by the Simons' stain method, required a lower protein loading per gram of glucan to obtain efficient hydrolysis compared with substrates containing less accessible cellulose. These results suggest that the rate-limiting step during hydrolysis is not the catalytic cleavage of the cellulose chains per se, but rather the limited accessibility of the enzymes to the cellulose chains due to the physical structure of the cellulosic substrate.
Figures



References
-
- Merino ST, Cherry J. Progress and challenges in enzyme development for biomass utilization. Adv Biochem Eng/Biotechnol. 2007;108:95–120. full_text. - PubMed
-
- Hogan CH, Mes-Hartree M, Saddler JN, Kushner D. Assessment of methods to determine minimal cellulase concentrations for efficient hydrolysis of cellulose. Appl Microbiol Biotechnol. 1990;32:614–620. doi: 10.1007/BF00173736. - DOI
-
- Mooney CA, Mansfield SD, Tuohy MG, Saddler JN. The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Biores Technol. 1998;64:113–119. doi: 10.1016/S0960-8524(97)00181-8. - DOI
LinkOut - more resources
Full Text Sources

