Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect
- PMID: 21310273
- PMCID: PMC3035713
- DOI: 10.1016/j.ajhg.2011.01.008
Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect
Abstract
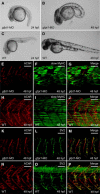
Neuromuscular junctions (NMJs) are synapses that transmit impulses from motor neurons to skeletal muscle fibers leading to muscle contraction. Study of hereditary disorders of neuromuscular transmission, termed congenital myasthenic syndromes (CMS), has helped elucidate fundamental processes influencing development and function of the nerve-muscle synapse. Using genetic linkage, we find 18 different biallelic mutations in the gene encoding glutamine-fructose-6-phosphate transaminase 1 (GFPT1) in 13 unrelated families with an autosomal recessive CMS. Consistent with these data, downregulation of the GFPT1 ortholog gfpt1 in zebrafish embryos altered muscle fiber morphology and impaired neuromuscular junction development. GFPT1 is the key enzyme of the hexosamine pathway yielding the amino sugar UDP-N-acetylglucosamine, an essential substrate for protein glycosylation. Our findings provide further impetus to study the glycobiology of NMJ and synapses in general.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kandel E.R., Schwartz J.H., Jessell T.M. McGraw-Hill; New York: 2000. Principles of Neural Science.
-
- McQuillen M.P. Familial limb-girdle myasthenia. Brain. 1966;89:121–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

