Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease
- PMID: 21310277
- PMCID: PMC3035706
- DOI: 10.1016/j.ajhg.2011.01.007
Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease
Abstract
The importance of intracellular folate metabolism is illustrated by the severity of symptoms and complications caused by inborn disorders of folate metabolism or by folate deficiency. We examined three children of healthy, distantly related parents presenting with megaloblastic anemia and cerebral folate deficiency causing neurologic disease with atypical childhood absence epilepsy. Genome-wide homozygosity mapping revealed a candidate region on chromosome 5 including the dihydrofolate reductase (DHFR) locus. DHFR sequencing revealed a homozygous DHFR mutation, c.458A>T (p.Asp153Val), in all siblings. The patients' folate profile in red blood cells (RBC), plasma, and cerebrospinal fluid (CSF), analyzed by liquid chromatography tandem mass spectrometry, was compatible with DHFR deficiency. DHFR activity and fluorescein-labeled methotrexate (FMTX) binding were severely reduced in EBV-immortalized lymphoblastoid cells of all patients. Heterozygous cells displayed intermediate DHFR activity and FMTX binding. RT-PCR of DHFR mRNA revealed no differences between wild-type and DHFR mutation-carrying cells, whereas protein expression was reduced in cells with the DHFR mutation. Treatment with folinic acid resulted in the resolution of hematological abnormalities, normalization of CSF folate levels, and improvement of neurological symptoms. In conclusion, the homozygous DHFR mutation p.Asp153Val causes DHFR deficiency and leads to a complex hematological and neurological disease that can be successfully treated with folinic acid. DHFR is necessary for maintaining sufficient CSF and RBC folate levels, even in the presence of adequate nutritional folate supply and normal plasma folate.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
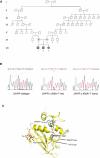

Comment in
-
Dihydrofolate reductase mutations-associated megaloblastic anemia and cerebral folate deficiency.Clin Genet. 2011 Jun;79(6):507-8. doi: 10.1111/j.1399-0004.2011.01662.x. Epub 2011 Apr 4. Clin Genet. 2011. PMID: 21388369 No abstract available.
References
-
- Stanger O. Physiology of folic acid in health and disease. Curr. Drug Metab. 2002;3:211–223. - PubMed
-
- Whitehead V.M. Acquired and inherited disorders of cobalamin and folate in children. Br. J. Haematol. 2006;134:125–136. - PubMed
-
- Tauro G.P., Danks D.M., Rowe P.B., Van der Weyden M.B., Schwarz M.A., Collins V.L., Neal B.W. Dihydrofolate reductase deficiency causing megaloblastic anemia in two families. N. Engl. J. Med. 1976;294:466–470. - PubMed
-
- Walters T.R. Congenital megaloblastic anemia responsive to N5-formyl tetrafolic acid administration. J. Pediatr. 1967;70:686–687.
-
- Erbe R.W. Inborn errors of folate metabolism (second of two parts) N. Engl. J. Med. 1975;293:807–812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

