The timing and duration of a sensitive period in human flavor learning: a randomized trial
- PMID: 21310829
- PMCID: PMC3076653
- DOI: 10.3945/ajcn.110.003541
The timing and duration of a sensitive period in human flavor learning: a randomized trial
Abstract
Background: By using the response to protein hydrolysate formula (PHF) as a model system, we discovered the existence of a sensitive period, before 4 mo, when exposure determines the hedonic tone to flavors.
Objective: We aimed to characterize the timing and duration of this sensitive period.
Design: Healthy infants, whose parents had chosen formula feeding, were randomly assigned into 1 of 6 groups at age 0.5 mo: 2 control groups, one fed cow milk-based formula (CMF) and the other fed PHF for 7 mo; 2 groups fed PHF for either 1 or 3 mo beginning at 1.5 mo and CMF otherwise; and 2 groups fed PHF for 1 mo beginning at either 2.5 or 3.5 mo and CMF otherwise. Brief access taste tests were conducted monthly, and complete "meals" of both formulas occurred at the end of the study.
Results: Three months of PHF exposure led to acceptance similar to that at 1 mo of exposure. Although these infants were more accepting than were infants with no exposure, they were less accepting than were infants with 7 mo of exposure, which suggests a dosing effect. The time when flavor experiences began was also significant. Among infants exposed to PHF for 1 mo, those who were first fed PHF at 3.5 mo rejected PHF relative to CMF more than did infants exposed at younger ages.
Conclusion: The general principles observed are likely of broader significance, indicating a fundamental feature of mammalian development and reflecting the importance of familiarizing infants with flavors that their mothers consume and transmit to breast milk. This trial was registered at clinicaltrials.gov as NCT00994747.
Figures


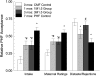
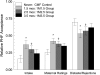

Comment in
-
Flavor exposure during sensitive periods of development as a key mechanism of flavor learning: implications for future research.Am J Clin Nutr. 2011 May;93(5):909-10. doi: 10.3945/ajcn.111.014381. Epub 2011 Apr 6. Am J Clin Nutr. 2011. PMID: 21471281 No abstract available.
References
-
- Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32(suppl 7):S62–71 - PubMed
-
- Mennella JA. The chemical senses and the development of flavor preferences in humans Hale TW, Hartmann PE, eds. Textbook on human lactation Amarillo, TX: Hale Publishing, 2007:403–14
-
- Bartoshuk LM, Beauchamp GK. Chemical senses. Annu Rev Psychol 1994;45:419–49 - PubMed

