A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy
- PMID: 21310918
- PMCID: PMC3109054
- DOI: 10.1167/iovs.10-6386
A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy
Abstract
Purpose: Although VEGF has been identified as an important mediator of the blood-retinal barrier alteration in diabetic retinopathy, the hypothesis for this study was that that other molecules, including the angiopoietins (Ang-1 and -2), may play a role. The expression of angiopoietins was analyzed in an animal model of diabetic retinopathy, and the role of Ang-2 in the regulation of diabetes-induced alterations of vascular permeability was characterized.
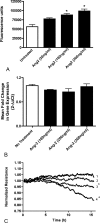
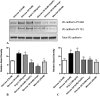
Methods: Diabetes was induced in rats, and human retinal endothelial cells (HRECs) were grown in media with 5.5 or 30.5 mM glucose. Levels of Ang-1 and -2 mRNA and protein were analyzed. Fluorescence-based assays were used to assess the effect of Ang-2 on vascular permeability in vivo and in vitro. The effect of Ang-2 on VE-cadherin function was assessed by measuring the extent of tyrosine phosphorylation.
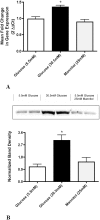
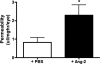
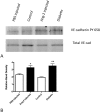
Results: Ang-2 mRNA and protein increased in the retinal tissues after 8 weeks of diabetes and in high-glucose-treated cells. Intravitreal injection of Ang-2 in rats produced a significant increase in retinal vascular permeability. Ang-2 increased HREC monolayer permeability that was associated with a decrease in VE-cadherin and a change in monolayer morphology. High glucose and Ang-2 produced a significant increase in VE-cadherin phosphorylation. CONCLUSIONS; Ang-2 is upregulated in the retina in an animal model of diabetes, and hyperglycemia induces the expression of Ang-2 in isolated retinal endothelial cells. Increased Ang-2 alters VE-cadherin function, leading to increased vascular permeability. Thus, Ang-2 may play an important role in increased vasopermeability in diabetic retinopathy.
Figures


References
-
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060 - PubMed
-
- Geiger B, Ayalon O. Cadherins Ann Rev Cell Biol. 1992;8:307–333 - PubMed
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–841 - PubMed
-
- Biester S, Ziemssen F, Ulrich Bartz-Schmidt K, Gelisken F. Is intravitreal bevacizumab treatment effective in diffuse diabetic macular edema? Graefes Arch Clin Exp Ophthalmol. 2009;247:1575–1577 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

