Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis
- PMID: 21311023
- PMCID: PMC3253482
- DOI: 10.1126/science.1199326
Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis
Abstract
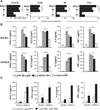
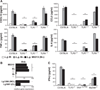
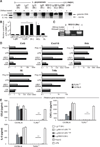
Mucocutaneous leishmaniasis is caused by infections with intracellular parasites of the Leishmania Viannia subgenus, including Leishmania guyanensis. The pathology develops after parasite dissemination to nasopharyngeal tissues, where destructive metastatic lesions form with chronic inflammation. Currently, the mechanisms involved in lesion development are poorly understood. Here we show that metastasizing parasites have a high Leishmania RNA virus-1 (LRV1) burden that is recognized by the host Toll-like receptor 3 (TLR3) to induce proinflammatory cytokines and chemokines. Paradoxically, these TLR3-mediated immune responses rendered mice more susceptible to infection, and the animals developed an increased footpad swelling and parasitemia. Thus, LRV1 in the metastasizing parasites subverted the host immune response to Leishmania and promoted parasite persistence.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

