Kelch repeat proteins control yeast PKA activity in response to nutrient availability
- PMID: 21311222
- PMCID: PMC3100789
- DOI: 10.4161/cc.10.5.14828
Kelch repeat proteins control yeast PKA activity in response to nutrient availability
Abstract
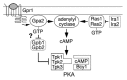
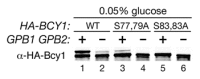
Regulation of protein kinase A (PKA) by binding of cAMP to the regulatory subunit and the resulting release of the active catalytic subunit is a very well established mechanism of kinase activation. We have shown recently that PKA in budding yeast is also subject to an additional level of regulation that that modulates its activity in response to nutrient availability. Nutrient regulation of PKA activity requires a pair of proteins, Gpb1 and Gpb2, that contain several kelch repeats, a sequence motif that predicts that they fold into a β-propeller structure. The regulatory process mediated by Gpb1 and Gpb2 causes an increase in the stability and phosphorylation of the PKA regulatory subunit Bcy1 in response to low extracellular glucose concentrations. Phosphorylation of serine-145 of Bcy1 controls its stability, and other phosphorylation events at the cluster of serines at positions 74-84 correlate with changes in nutrient availability. Here we present data consistent with a model in which the effects of Gpb1 and Gpb2 on Bcy1 are an indirect consequence of their primary effects on the PKA catalytic subunits.
© 2011 Landes Bioscience
Figures
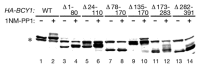
References
-
- Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, et al. Dynamics of signaling by PKA. Biochim Biophys Acta. 2005;1754:25–37. - PubMed
-
- Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–3765. - PubMed
-
- Rubio-Texeira M, Van Zeebroeck G, Voordeckers K, Thevelein JM. Saccharomyces cerevisiae plasma membrane nutrient sensors and their role in PKA signaling. FEMS Yeast Res. 2009;10:134–149. - PubMed
-
- Kraakman L, Lemaire K, Ma P, Teunissen AWRH, Donaton MCV, Van Dijck P, et al. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32:1002–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
