Use of the Ages and Stages Questionnaire and Bayley Scales of Infant Development-II in neurodevelopmental follow-up of extremely low birth weight infants
- PMID: 21311498
- PMCID: PMC3139816
- DOI: 10.1038/jp.2011.1
Use of the Ages and Stages Questionnaire and Bayley Scales of Infant Development-II in neurodevelopmental follow-up of extremely low birth weight infants
Abstract
Objective: For infants born with extremely low birth weight (ELBW), we examined the (1) correlation between results on the Ages and Stages Questionnaire (ASQ) and the Bayley Scales of Infant Development-II (BSID-II) at 18 to 22 months corrected age; (2) degree to which earlier ASQ assessments predict later BSID-II results; (3) impact of ASQ use on follow-up study return rates.
Study design: ASQ data were collected at 4, 8, 12 and 18 to 22 months corrected age. The BSID-II was completed at 18 to 22 months corrected age. ASQ and BSID-II 18 to 22 month sensitivity and specificity were examined. Ability of earlier ASQs to predict later BSID-II scores was examined through linear regression analyses.
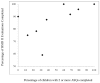
Result: ASQ sensitivity and specificity at 18 to 22 months were 73 and 65%, respectively. Moderate correlation existed between earlier ASQ and later BSID-II results.
Conclusion: For extremely low birth weight infant assessment, the ASQ cannot substitute for the BSID-II, but seems to improve tracking success.
Figures
References
-
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in national institute of child health and human development neonatal research network, 1993-1994. Pediatrics. 2000;105:1216–1225. - PubMed
-
- Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, et al. Neurodevelopmental outcome and growth at 18-22 months corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114:1287–1291. - PubMed
-
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007;120:40–48. - PubMed
-
- Hille ET, Elbertse L, Grovenhorst JB, Brand R, Verloove-Vanhorickk SP, Dutch POPS-19 Collaborative Study Group Nonresponse bias in a follow-up study of 19-year-old adolescents born as preterm infants. Pediatrics. 2005;116(5):e662–666. - PubMed
-
- Aylward GP. Methodological issues in outcome studies of at risk infants. J Pediatr Psychol. 2002;27:37–45. - PubMed