Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins
- PMID: 21311561
- PMCID: PMC3059911
- DOI: 10.1038/embor.2011.5
Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins
Abstract
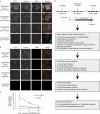
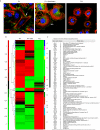
A characteristic of integrins is their ability to transfer chemical and mechanical signals across the plasma membrane. Force generated by myosin II makes cells able to sense substrate stiffness and induce maturation of nascent adhesions into focal adhesions. In this paper, we present a comprehensive proteomic analysis of nascent and mature adhesions. The purification of integrin adhesion complexes combined with quantitative mass spectrometry enabled the identification and quantification of known and new adhesion-associated proteins. Furthermore, blocking adhesion maturation with the myosin II inhibitor blebbistatin markedly impaired the recruitment of LIM domain proteins to integrin adhesion sites. This suggests a common recruitment mechanism for a whole class of adhesion-associated proteins, involving myosin II and the zinc-finger-type LIM domain.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Bear JE et al. (2002) Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109: 509–521 - PubMed
-
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE (1998) Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature 392: 730–733 - PubMed
-
- Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EH (2009) Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci 122: 1665–1679 - PubMed
-
- de Hoog CL, Foster LJ, Mann M (2004) RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell 117: 649–662 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

