Changing patterns in long-acting bronchodilator trials in chronic obstructive pulmonary disease
- PMID: 21311692
- PMCID: PMC3034288
- DOI: 10.2147/COPD.S14680
Changing patterns in long-acting bronchodilator trials in chronic obstructive pulmonary disease
Abstract
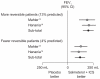
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. Developments in the understanding of COPD have led to standard guidelines for diagnosis, treatment, and spirometry assessments, which have in turn influenced trial designs and inclusion criteria. Substantial clinical evidence has been gained from clinical trials and supports a positive approach to COPD management. However, there appear to be changing trends in recent trials. Large bronchodilator studies have reported lower improvements in trough forced expiratory volume in 1 second (FEV(1)) values versus placebo than were observed in earlier studies, while the rate of FEV(1) decline seems to be lower in more recent trials. In addition, recent evidence has called into question the usefulness of bronchodilator reversibility testing as a trial inclusion criterion. Baseline patient populations and use of concomitant medications have also changed over recent years due to increased treatment options. The impact of these many variables on clinical trial results is explored, with a particular focus on changes in inclusion criteria and patient baseline demographics.
Keywords: chronic obstructive pulmonary disease; clinical trials; forced expiratory volume in 1 second; long-acting bronchodilators; lung function.
Figures


References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Accessed 2010 Jan 13]. Updated 2009. Available from: http://www.goldcopd.com.
-
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. - PubMed
-
- Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. - PubMed
-
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. - PubMed
-
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

