Interferon-α induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZB×NZW)F1 mice but not in BALB/c mice
- PMID: 21312191
- PMCID: PMC3073415
- DOI: 10.1002/eji.201040649
Interferon-α induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZB×NZW)F1 mice but not in BALB/c mice
Abstract
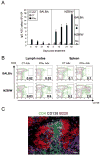
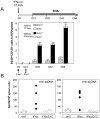
IFN-α is known to play a critical role in the pathogenesis of systemic lupus erythematosus (SLE), but the mechanisms remain unclear. We previously showed that within weeks, exposure to IFN-α in vivo induces lupus in pre-autoimmune lupus-prone NZB×NZW F1 (NZB/W) but not in BALB/c mice. In the current study, we show that in vivo expression of IFN-α induces sustained B-cell proliferation in both BALB/c and NZB/W mice. In NZB/W but not BALB/c mice, B-cell proliferation was accompanied by a rapid and unabated production of autoantibody-secreting cells (ASCs) in secondary lymphoid organs, suggesting that a B-cell checkpoint is altered in the autoimmune background. The majority (>95%) of ASCs elicited in IFN-α-treated NZB/W mice were short-lived and occurred without the induction of long-lived plasma cells. A short course of cyclophosphamide caused a sharp drop in IFN-α-elicited short-lived plasma cells, but the levels recovered within days following termination of treatment. Thus, our work provides new insights into effectiveness and limitations of the current SLE therapies.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures



Comment in
-
Plasma cells in systemic lupus erythematosus: the long and short of it all.Eur J Immunol. 2011 Mar;41(3):588-91. doi: 10.1002/eji.201041354. Epub 2011 Feb 10. Eur J Immunol. 2011. PMID: 21341259
References
-
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. - PubMed
-
- Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. - PubMed
-
- Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. - PubMed
-
- Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

