Actin structure and function
- PMID: 21314430
- PMCID: PMC3130349
- DOI: 10.1146/annurev-biophys-042910-155359
Actin structure and function
Abstract
Actin is the most abundant protein in most eukaryotic cells. It is highly conserved and participates in more protein-protein interactions than any known protein. These properties, along with its ability to transition between monomeric (G-actin) and filamentous (F-actin) states under the control of nucleotide hydrolysis, ions, and a large number of actin-binding proteins, make actin a critical player in many cellular functions, ranging from cell motility and the maintenance of cell shape and polarity to the regulation of transcription. Moreover, the interaction of filamentous actin with myosin forms the basis of muscle contraction. Owing to its central role in the cell, the actin cytoskeleton is also disrupted or taken over by numerous pathogens. Here we review structures of G-actin and F-actin and discuss some of the interactions that control the polymerization and disassembly of actin.
Figures



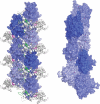
References
-
- Barth H, Stiles BG. Binary actin-ADP-ribosylating toxins and their use as molecular Trojan horses for drug delivery into eukaryotic cells. Curr. Med. Chem. 2008;15:459–-69. - PubMed
-
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, et al. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell. 1997;90:661–-70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

