BSE infectivity in jejunum, ileum and ileocaecal junction of incubating cattle
- PMID: 21314904
- PMCID: PMC3048543
- DOI: 10.1186/1297-9716-42-21
BSE infectivity in jejunum, ileum and ileocaecal junction of incubating cattle
Abstract
To establish bovine spongiform encephalopathy (BSE) public health protection measures it is important to precisely define the cattle tissues considered as specified risk materials (SRM). To date, in pre-clinical BSE infected cattle, no evidence of the BSE agent had been found in the gut outside of the ileal Peyer's Patches. This study was undertaken to determine when and where the pathological prion protein (PrPSc) and/or BSE infectivity can be found in the small intestine of cattle 4 to 6 months of age, orally challenged with BSE. Samples of the jejunum, the ileum and the ileocaecal junction from 46 BSE infected cattle, culled from 1 up to 44 months post infection (mpi) were examined by immunohistochemistry. Samples from cattle 8 mpi to 20 mpi were additionally studied by PTA Western blot, rapid tests, and by mouse (TgbovXV) bioassay. In doing so nearly all of the cattle, from 4 up to 44 mpi, had detectable amounts of PrPSc and/or infectivity in the distal ileum. In the distal ileum clear time-dependent variations were visible concerning the amount of PrPSc, the tissue structures affected, and the cells involved. BSE infectivity was found not only in the ileum and ileocaecal junction but also in the jejunum. The systematic approach of this study provides new data for qualitative and quantitative risk assessments and allows defining bovine SRM more precisely.
Figures



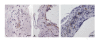
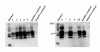
Similar articles
-
Complementary studies detecting classical bovine spongiform encephalopathy infectivity in jejunum, ileum and ileocaecal junction in incubating cattle.Vet Res. 2013 Dec 21;44(1):123. doi: 10.1186/1297-9716-44-123. Vet Res. 2013. PMID: 24359408 Free PMC article.
-
Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy.Vet Rec. 2003 Mar 29;152(13):387-92. doi: 10.1136/vr.152.13.387. Vet Rec. 2003. PMID: 12696704
-
Detection of disease-associated prion protein in the posterior portion of the small intestine involving the continuous Peyer's patch in cattle orally infected with bovine spongiform encephalopathy agent.Transbound Emerg Dis. 2011 Aug;58(4):333-43. doi: 10.1111/j.1865-1682.2011.01208.x. Epub 2011 Feb 14. Transbound Emerg Dis. 2011. PMID: 21320296
-
Pathogenesis of classical and atypical BSE in cattle.Prev Vet Med. 2011 Nov 1;102(2):112-7. doi: 10.1016/j.prevetmed.2011.04.006. Epub 2011 May 17. Prev Vet Med. 2011. PMID: 21592603 Review.
-
Bovine spongiform encephalopathy.J Am Vet Med Assoc. 2009 Jan 1;234(1):59-72. doi: 10.2460/javma.234.1.59. J Am Vet Med Assoc. 2009. PMID: 19119967 Review.
Cited by
-
Intra- and Interspecies Transmission of Atypical BSE - What Can We Learn from It?Food Saf (Tokyo). 2016 Dec 22;4(4):121-129. doi: 10.14252/foodsafetyfscj.2016023. eCollection 2016 Dec. Food Saf (Tokyo). 2016. PMID: 32231916 Free PMC article. Review.
-
Comparative analysis of prions in nervous and lymphoid tissues of chronic wasting disease-infected cervids.J Gen Virol. 2018 May;99(5):753-758. doi: 10.1099/jgv.0.001053. Epub 2018 Mar 26. J Gen Virol. 2018. PMID: 29580373 Free PMC article.
-
The diverse roles of mononuclear phagocytes in prion disease pathogenesis.Prion. 2012 Apr-Jun;6(2):124-33. doi: 10.4161/pri.18853. Epub 2012 Apr 1. Prion. 2012. PMID: 22421209 Free PMC article. Review.
-
Bovine spongiform encephalopathy (BSE) cases born after the total feed ban.EFSA J. 2017 Jul 13;15(7):e04885. doi: 10.2903/j.efsa.2017.4885. eCollection 2017 Jul. EFSA J. 2017. PMID: 32625550 Free PMC article.
-
Proteinase K-resistant material in ARR/VRQ sheep brain affected with classical scrapie is composed mainly of VRQ prion protein.J Virol. 2011 Dec;85(23):12537-46. doi: 10.1128/JVI.00448-11. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917981 Free PMC article.
References
-
- Hoffmann C, Ziegler U, Buschmann A, Weber A, Kupfer L, Oelschlegel A, Hammerschmidt B, Groschup MH. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J Gen Virol. 2007;88:1048–1055. doi: 10.1099/vir.0.82186-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

