Evaluation of immune responses following infection of ponies with an EHV-1 ORF1/2 deletion mutant
- PMID: 21314906
- PMCID: PMC3045331
- DOI: 10.1186/1297-9716-42-23
Evaluation of immune responses following infection of ponies with an EHV-1 ORF1/2 deletion mutant
Abstract
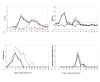
Equine herpesvirus-1 (EHV-1) infection remains a significant problem despite the widespread use of vaccines. The inability to generate a protective immune response to EHV-1 vaccination or infection is thought to be due to immunomodulatory properties of the virus, and the ORF1 and ORF2 gene products have been hypothesized as potential candidates with immunoregulatory properties. A pony infection study was performed to define immune responses to EHV-1, and to determine if an EHV-1 ORF1/2 deletion mutant (ΔORF1/2) would have different disease and immunoregulatory effects compared to wild type EHV-1 (WT). Infection with either virus led to cytokine responses that coincided with the course of clinical disease, particularly the biphasic pyrexia, which correlates with respiratory disease and viremia, respectively. Similarly, both viruses caused suppression of proliferative T-cell responses on day 7 post infection (pi). The ΔORF1/ORF2 virus caused significantly shorter primary pyrexia and significantly reduced nasal shedding, and an attenuated decrease in PBMC IL-8 as well as increased Tbet responses compared to WT-infected ponies. In conclusion, our findings are (i) that infection of ponies with EHV-1 leads to modulation of immune responses, which are correlated with disease pathogenesis, and (ii) that the ORF1/2 genes are of importance for disease outcome and modulation of cytokine responses.
Figures


Similar articles
-
Deletion of the ORF2 gene of the neuropathogenic equine herpesvirus type 1 strain Ab4 reduces virulence while maintaining strong immunogenicity.BMC Vet Res. 2018 Aug 22;14(1):245. doi: 10.1186/s12917-018-1563-4. BMC Vet Res. 2018. PMID: 30134896 Free PMC article.
-
The deletion of the ORF1 and ORF71 genes reduces virulence of the neuropathogenic EHV-1 strain Ab4 without compromising host immunity in horses.PLoS One. 2018 Nov 15;13(11):e0206679. doi: 10.1371/journal.pone.0206679. eCollection 2018. PLoS One. 2018. PMID: 30440016 Free PMC article.
-
Intranasal IgG4/7 antibody responses protect horses against equid herpesvirus-1 (EHV-1) infection including nasal virus shedding and cell-associated viremia.Virology. 2019 May;531:219-232. doi: 10.1016/j.virol.2019.03.014. Epub 2019 Mar 22. Virology. 2019. PMID: 30928700
-
Immune escape of equine herpesvirus 1 and other herpesviruses of veterinary importance.Vet Immunol Immunopathol. 2006 May 15;111(1-2):31-40. doi: 10.1016/j.vetimm.2006.01.006. Epub 2006 Feb 10. Vet Immunol Immunopathol. 2006. PMID: 16472872 Review.
-
Viremia and nasal shedding for the diagnosis of equine herpesvirus-1 infection in domesticated horses.J Vet Intern Med. 2024 May-Jun;38(3):1765-1791. doi: 10.1111/jvim.16958. Epub 2023 Dec 9. J Vet Intern Med. 2024. PMID: 38069548 Free PMC article. Review.
Cited by
-
Comparative Genomic Sequencing and Pathogenic Properties of Equine Herpesvirus 1 KyA and RacL11.Front Vet Sci. 2017 Dec 11;4:211. doi: 10.3389/fvets.2017.00211. eCollection 2017. Front Vet Sci. 2017. PMID: 29312962 Free PMC article.
-
Major histocompatibility complex class I downregulation induced by equine herpesvirus type 1 pUL56 is through dynamin-dependent endocytosis.J Virol. 2014 Nov;88(21):12802-15. doi: 10.1128/JVI.02079-14. Epub 2014 Aug 27. J Virol. 2014. PMID: 25165105 Free PMC article.
-
A Live-Attenuated Equine Influenza Vaccine Stimulates Innate Immunity in Equine Respiratory Epithelial Cell Cultures That Could Provide Protection From Equine Herpesvirus 1.Front Vet Sci. 2021 Jun 10;8:674850. doi: 10.3389/fvets.2021.674850. eCollection 2021. Front Vet Sci. 2021. PMID: 34179166 Free PMC article.
-
Impact of the host immune response on the development of equine herpesvirus myeloencephalopathy in horses.J Gen Virol. 2024 May;105(5):001987. doi: 10.1099/jgv.0.001987. J Gen Virol. 2024. PMID: 38767608 Free PMC article.
-
Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions.Vet Res. 2013 Dec 5;44(1):118. doi: 10.1186/1297-9716-44-118. Vet Res. 2013. PMID: 24308772 Free PMC article.
References
-
- Allen GP, Kydd JH, Slater JD, Smith KC. In: Infectious Diseases of Livestock. 1. Coetzer JAWT, RC, editor. Vol. 2. Newmarket: Oxford University Press; 2004. Equid herpesvirus 1 and equid herpesvirus 4 infections; pp. 829–859.
-
- Kydd JH, Wattrang E, Hannant D. Pre-infection frequencies of equine herpesvirus-1 specific, cytotoxic T lymphocytes correlate with protection against abortion following experimental infection of pregnant mares. Vet Immunol Immunopathol. 2003;96(3-4):207–217. doi: 10.1016/j.vetimm.2003.08.004. - DOI - PubMed
-
- Soboll G, Horohov DW, Aldridge BM, Olsen CW, McGregor MW, Drape RJ, Macklin MD, Swain WF, Lunn DP. Regional antibody and cellular immune responses to equine influenza virus infection, and particle mediated DNA vaccination. Vet Immunol Immunopathol. 2003;94(1-2):47–62. doi: 10.1016/S0165-2427(03)00060-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

