Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial
- PMID: 21314924
- PMCID: PMC3045390
- DOI: 10.1186/1471-2466-11-8
Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial
Abstract
Background: Bronchial thermoplasty (BT) is a bronchoscopic procedure that improves asthma control by reducing excess airway smooth muscle. Treated patients have been followed out to 5 years to evaluate long-term safety of this procedure.
Methods: Patients enrolled in the Asthma Intervention Research Trial were on inhaled corticosteroids ≥200 μg beclomethasone or equivalent + long-acting-beta2-agonists and demonstrated worsening of asthma on long-acting-β2-agonist withdrawal. Following initial evaluation at 1 year, subjects were invited to participate in a 4 year safety study. Adverse events (AEs) and spirometry data were used to assess long-term safety out to 5 years post-BT.
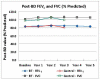
Results: 45 of 52 treated and 24 of 49 control group subjects participated in long-term follow-up of 5 years and 3 years respectively. The rate of respiratory adverse events (AEs/subject) was stable in years 2 to 5 following BT (1.2, 1.3, 1.2, and 1.1, respectively,). There was no increase in hospitalizations or emergency room visits for respiratory symptoms in Years 2, 3, 4, and 5 compared to Year 1. The FVC and FEV1 values showed no deterioration over the 5 year period in the BT group. Similar results were obtained for the Control group.
Conclusions: The absence of clinical complications (based on AE reporting) and the maintenance of stable lung function (no deterioration of FVC and FEV1) over a 5-year period post-BT in this group of patients with moderate to severe asthma support the long-term safety of the procedure out to 5 years.
Figures
References
-
- National Health Interview Survey, National Center for Health Statistics. CDC. 2008. http://www.cdc.gov/nchs/fastats/asthma.htm Accessed 2/10/10.
-
- Schatz M, Zeiger R, Mosen D, Vollmer W. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care. 2008;14:206–211. - PubMed
-
- Vollmer W, Markson L, O'Connor E, Frazier E, Berger M, Buist AS. Association of Asthma Control with Health Care Utilization - A Prospective Evaluation. Am J Resp Crit Care Med. 2002;165:195–199. - PubMed