Malaria rapid diagnostic kits: quality of packaging, design and labelling of boxes and components and readability and accuracy of information inserts
- PMID: 21314992
- PMCID: PMC3045995
- DOI: 10.1186/1475-2875-10-39
Malaria rapid diagnostic kits: quality of packaging, design and labelling of boxes and components and readability and accuracy of information inserts
Abstract
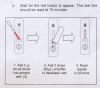
Background: The present study assessed malaria RDT kits for adequate and correct packaging, design and labelling of boxes and components. Information inserts were studied for readability and accuracy of information.
Methods: Criteria for packaging, design, labelling and information were compiled from Directive 98/79 of the European Community (EC), relevant World Health Organization (WHO) documents and studies on end-users' performance of RDTs. Typography and readability level (Flesch-Kincaid grade level) were assessed.
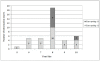
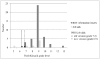
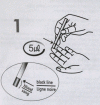
Results: Forty-two RDT kits from 22 manufacturers were assessed, 35 of which had evidence of good manufacturing practice according to available information (i.e. CE-label affixed or inclusion in the WHO list of ISO13485:2003 certified manufacturers). Shortcomings in devices were (i) insufficient place for writing sample identification (n=40) and (ii) ambiguous labelling of the reading window (n=6). Buffer vial labels were lacking essential information (n=24) or were of poor quality (n=16). Information inserts had elevated readability levels (median Flesch Kincaid grade 8.9, range 7.1-12.9) and user-unfriendly typography (median font size 8, range 5-10). Inadequacies included (i) no referral to biosafety (n=18), (ii) critical differences between depicted and real devices (n=8), (iii) figures with unrealistic colours (n=4), (iv) incomplete information about RDT line interpretations (n=31) and no data on test characteristics (n=8). Other problems included (i) kit names that referred to Plasmodium vivax although targeting a pan-species Plasmodium antigen (n=4), (ii) not stating the identity of the pan-species antigen (n=2) and (iii) slight but numerous differences in names displayed on boxes, device packages and information inserts. Three CE labelled RDT kits produced outside the EC had no authorized representative affixed and the shape and relative dimensions of the CE symbol affixed did not comply with the Directive 98/79/EC. Overall, RDTs with evidence of GMP scored better compared to those without but inadequacies were observed in both groups.
Conclusion: Overall, malaria RDTs showed shortcomings in quality of construction, design and labelling of boxes, device packages, devices and buffers. Information inserts were difficult to read and lacked relevant information.
Figures








Similar articles
-
Self-diagnosis of malaria by travelers and expatriates: assessment of malaria rapid diagnostic tests available on the internet.PLoS One. 2013;8(1):e53102. doi: 10.1371/journal.pone.0053102. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301027 Free PMC article.
-
Quality issues with malaria rapid diagnostic test accessories and buffer packaging: findings from a 5-country private sector project in Africa.Malar J. 2017 Apr 20;16(1):160. doi: 10.1186/s12936-017-1820-1. Malar J. 2017. PMID: 28427428 Free PMC article.
-
Harmonization of malaria rapid diagnostic tests: best practices in labelling including instructions for use.Malar J. 2014 Dec 17;13:505. doi: 10.1186/1475-2875-13-505. Malar J. 2014. PMID: 25519980 Free PMC article.
-
Malaria rapid diagnostic tests in endemic settings.Clin Microbiol Infect. 2013 May;19(5):399-407. doi: 10.1111/1469-0691.12151. Epub 2013 Feb 25. Clin Microbiol Infect. 2013. PMID: 23438048 Review.
-
[Rapid diagnostic test for malaria].Bull Soc Pathol Exot. 2017 Feb;110(1):49-54. doi: 10.1007/s13149-017-0549-y. Epub 2017 Feb 7. Bull Soc Pathol Exot. 2017. PMID: 28176239 Review. French.
Cited by
-
Evaluation of MicroScan Bacterial Identification Panels for Low-Resource Settings.Diagnostics (Basel). 2021 Feb 19;11(2):349. doi: 10.3390/diagnostics11020349. Diagnostics (Basel). 2021. PMID: 33669829 Free PMC article.
-
Transforming Rapid Diagnostic Tests for Precision Public Health: Open Guidelines for Manufacturers and Users.JMIR Biomed Eng. 2022 Jul 29;7(2):e26800. doi: 10.2196/26800. JMIR Biomed Eng. 2022. PMID: 38875688 Free PMC article.
-
Assessment of the knowledge of graphical symbols labelled on malaria rapid diagnostic tests in four international settings.Malar J. 2011 Nov 2;10:331. doi: 10.1186/1475-2875-10-331. Malar J. 2011. PMID: 22047089 Free PMC article.
-
Evaluation of the rapid diagnostic test CareStart pLDH Malaria (Pf-pLDH/pan-pLDH) for the diagnosis of malaria in a reference setting.Malar J. 2012 Jun 18;11:204. doi: 10.1186/1475-2875-11-204. Malar J. 2012. PMID: 22704733 Free PMC article.
-
Self-diagnosis of malaria by travelers and expatriates: assessment of malaria rapid diagnostic tests available on the internet.PLoS One. 2013;8(1):e53102. doi: 10.1371/journal.pone.0053102. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301027 Free PMC article.
References
-
- World Health Organization. Guidelines for the treatment of malaria. second. 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf - PubMed
-
- Chiodini PL, Bowers K, Jorgensen P, Barnwell JW, Grady KK, Luchavez J, Moody AH, Cenizal A, Bell D. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg. 2007;101:331–337. doi: 10.1016/j.trstmh.2006.09.007. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

