The L76V mutation in HIV-1 protease is potentially associated with hypersusceptibility to protease inhibitors Atazanavir and Saquinavir: is there a clinical advantage?
- PMID: 21314993
- PMCID: PMC3049128
- DOI: 10.1186/1742-6405-8-7
The L76V mutation in HIV-1 protease is potentially associated with hypersusceptibility to protease inhibitors Atazanavir and Saquinavir: is there a clinical advantage?
Abstract
Background: Although being considered as a rarely observed HIV-1 protease mutation in clinical isolates, the L76V-prevalence increased 1998-2008 in some European countries most likely due to the approval of Lopinavir, Amprenavir and Darunavir which can select L76V. Beside an enhancement of resistance, L76V is also discussed to confer hypersusceptibility to the drugs Atazanavir and Saquinavir which might enable new treatment strategies by trying to take advantage of particular mutations.
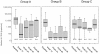
Results: Based on a cohort of 47 L76V-positive patients, we examined if there might exist a clinical advantage for L76V-positive patients concerning long-term success of PI-containing regimens in patients with limited therapy options.Genotypic- and phenotypic HIV-resistance tests from 47 mostly multi-resistant, L76V-positive patients throughout Germany were accomplished retrospectively 1999-2009. Five genotype-based drug-susceptibility predictions received from online interpretation-tools for Atazanavir, Saquinavir, Amprenavir and Lopinavir, were compared to phenotype-based predictions that were determined by using a recombinant virus assay along with a Virtual Phenotype™(Virco). The clinical outcome of the L76V-adapted follow-up therapy was determined by monitoring viral load for 96 weeks.
Conclusions: In this analysis, the mostly used interpretation systems overestimated the L76V-mutation concerning Atazanavir- and SQV resistance. In fact, a clear benefit in drug susceptibility for these drugs was observed in phenotype analysis after establishment of L76V. More importantly, long-term therapy success was significantly higher in patients receiving Atazanavir and/or Saquinavir plus one L76V-selecting drug compared to patients without L76V-selecting agents (p = 0.002).In case of L76V-occurrence ATV and/or SQV may represent encouraging options for patients in deep salvage situations.
Figures


Similar articles
-
Prevalence, mutation patterns, and effects on protease inhibitor susceptibility of the L76V mutation in HIV-1 protease.Antimicrob Agents Chemother. 2010 Nov;54(11):4903-6. doi: 10.1128/AAC.00906-10. Epub 2010 Aug 30. Antimicrob Agents Chemother. 2010. PMID: 20805393 Free PMC article.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
-
Performance of six different ritonavir-boosted protease inhibitor-based regimens in heavily antiretroviral-experienced HIV-infected patients.HIV Clin Trials. 2006 Jul-Aug;7(4):163-71. doi: 10.1310/hct0704-163. HIV Clin Trials. 2006. PMID: 17065028
-
Prevalence of darunavir resistance mutations in HIV-1-infected patients failing other protease inhibitors.J Antimicrob Chemother. 2007 Oct;60(4):885-8. doi: 10.1093/jac/dkm276. Epub 2007 Jul 23. J Antimicrob Chemother. 2007. PMID: 17646201
-
First-line boosted protease inhibitor-based regimens in treatment-naive HIV-1-infected patients--making a good thing better.AIDS Rev. 2009 Oct-Dec;11(4):215-22. AIDS Rev. 2009. PMID: 19940948 Review.
Cited by
-
Collinearity of protease mutations in HIV-1 samples with high-level protease inhibitor class resistance.J Antimicrob Chemother. 2013 Feb;68(2):414-8. doi: 10.1093/jac/dks409. Epub 2012 Oct 19. J Antimicrob Chemother. 2013. PMID: 23085775 Free PMC article.
-
Potent antiviral HIV-1 protease inhibitor GRL-02031 adapts to the structures of drug resistant mutants with its P1'-pyrrolidinone ring.J Med Chem. 2012 Apr 12;55(7):3387-97. doi: 10.1021/jm300072d. Epub 2012 Mar 22. J Med Chem. 2012. PMID: 22401672 Free PMC article.
-
Prototypical Recombinant Multi-Protease-Inhibitor-Resistant Infectious Molecular Clones of Human Immunodeficiency Virus Type 1.Antimicrob Agents Chemother. 2013 Sep;57(9):4290-4299. doi: 10.1128/AAC.00614-13. Epub 2013 Jun 24. Antimicrob Agents Chemother. 2013. PMID: 23796938 Free PMC article.
-
Non-active site mutants of HIV-1 protease influence resistance and sensitisation towards protease inhibitors.Retrovirology. 2020 May 19;17(1):13. doi: 10.1186/s12977-020-00520-6. Retrovirology. 2020. PMID: 32430025 Free PMC article.
-
Description of the L76V resistance protease mutation in HIV-1 B and "non-B" subtypes.PLoS One. 2013;8(1):e54381. doi: 10.1371/journal.pone.0054381. Epub 2013 Jan 18. PLoS One. 2013. PMID: 23349869 Free PMC article.
References
-
- Catucci M, Venturi G, Romano L, Riccio ML, De Milito A, Valensin PE, Zazzi M. Development and significance of the HIV-1 reverse transcriptase M184V mutation during combination therapy with lamivudine, zidovudine, and protease inhibitors. J Acquir Immune Defic Syndr. 1999;21(3):203–8. - PubMed
-
- Masquelier B, Descamps D, Carriere I, Ferchal F, Collin G, Denayrolles M, Ruffault A, Chanzy B, Izopet J, Buffet-Janvresse C, Schmitt MP, Race E, Fleury HJ, Aboulker JP, Yeni P, Brun-Vézinet F. Zidovudine resensitization and dual HIV-1 resistance to zidovudine and lamivudine in the delta lamivudine roll-over study. Antivir Ther. 1999;4(2):69–77. - PubMed
-
- Matamoros T, Franco S, Vazquez-Alvarez BM, Mas A, Martinez MA, Mendez-Arias L. Molecular determinants of multi-nucleoside analogue resistance in HIV-1 reverse transcriptases containing a dipeptide insertion in the fingers subdomain: effect of mutations D67N and T215Y on removal of thymidine nucleotide analogues from blocked DNA primers. J Biol Chem. 2004;279(23):24569–77. doi: 10.1074/jbc.M312658200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

