Hyaluronan suppresses prostate tumor cell proliferation through diminished expression of N-cadherin and aberrant growth factor receptor signaling
- PMID: 21315068
- PMCID: PMC3070779
- DOI: 10.1016/j.yexcr.2011.01.026
Hyaluronan suppresses prostate tumor cell proliferation through diminished expression of N-cadherin and aberrant growth factor receptor signaling
Abstract
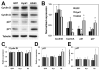
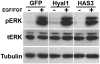
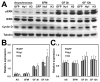
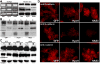
Hyaluronan (HA) production has been functionally implicated in prostate tumorigenesis and metastasis. We previously used prostate tumor cells overexpressing the HA synthesizing enzyme HAS3 or the clinically relevant hyaluronidase Hyal1 to show that excess HA production suppresses tumor growth, while HA turnover accelerates spontaneous metastasis from the prostate. Here, we examined pathways responsible for effects of HAS3 and Hyal1 on tumor cell phenotype. Detailed characterization of cell cycle progression revealed that expression of Hyal1 accelerated cell cycle re-entry following synchronization, whereas HAS3 alone delayed entry. Hyal1 expressing cells exhibited a significant reduction in their ability to sustain ERK phosphorylation upon stimulation by growth factors, and in their expression of the cyclin-dependent kinase inhibitor p21. In contrast, HAS3 expressing cells showed prolonged ERK phosphorylation and increased expression of both p21 and p27, in asynchronous and synchronized cultures. Changes in cell cycle regulatory proteins were accompanied by HA-induced suppression of N-cadherin, while E-cadherin expression and β-catenin expression and distribution remained unchanged. Our results are consistent with a model in which excess HA synthesis suppresses cell proliferation by promoting homotypic E-cadherin mediated cell-cell adhesion, consequently signaling to elevate cell cycle inhibitor expression and suppress G1- to S-phase transition.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. - PubMed
-
- Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998;58:342–347. - PubMed
-
- Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–11932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

