Obesity surgery and gut-brain communication
- PMID: 21315095
- PMCID: PMC3118403
- DOI: 10.1016/j.physbeh.2011.01.023
Obesity surgery and gut-brain communication
Abstract
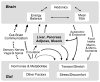
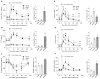
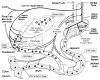
The prevalence of obesity, and the cluster of serious metabolic diseases it is associated with, continues to rise globally, and hopes for effective treatment with drugs have been considerably set back. Thus, success with bariatric surgeries to induce sustained body weight loss and effectively cure most of the associated co-morbidities appears almost "miraculous" and systematic investigation of the mechanisms at work has gained momentum. Here, we will discuss the basic organization of gut-brain communication and review clinical and pre-clinical investigations on the potential mechanisms by which gastric bypass surgery leads to its beneficial effects on energy balance and glucose homeostasis. Although a lot has been learned regarding changes in energy intake and expenditure, secretion of gut hormones, and improvement in glucose homeostasis, there has not yet been the "breakthrough observation" of identifying a key signaling component common to the beneficial effects of the surgery. However, given the complexity and redundancy of gut-brain signaling and gut signaling to other relevant organs, it is perhaps more realistic to expect a number of key signaling changes that act in concert to bring about the "miracle".
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

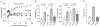
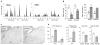
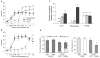
References
-
- Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15(1):216–24. - PubMed
-
- Koopmans HS. Internal signals cause large changes in food intake in one-way crossed intestines rats. Brain Res Bull. 1985;14(6):595–603. - PubMed
-
- Koopmans HS. An integrated organismic response to lower gut stimulation. Scand J Gastroenterol Suppl. 1983;82:143–53. - PubMed
-
- Koopmans HS, Sclafani A. Control of body weight by lower gut signals. Int J Obes. 1981;5(5):491–5. - PubMed
-
- Koopmans HS, Sclafani A, Fichtner C, Aravich PF. The effects of ileal transposition on food intake and body weight loss in VMH-obese rats. Am J Clin Nutr. 1982;35(2):284–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

