Development and cancer of the cerebellum
- PMID: 21315459
- PMCID: PMC3051031
- DOI: 10.1016/j.tins.2011.01.002
Development and cancer of the cerebellum
Abstract
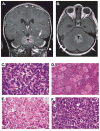
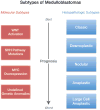
Medulloblastoma (MB) is the most common malignant pediatric brain tumor and is thought to arise from genetic anomalies in developmental pathways required for the normal maturation of the cerebellar cortex, notably developmental pathways for granule cell progenitor (GCP) neurogenesis. Over the past decade, a wide range of studies have identified genes and their regulators within signaling pathways, as well as noncoding RNAs, that have crucial roles in both normal cerebellar development and pathogenesis. These include the Notch, Wnt/β-catenin, bone morphogenic proteins (Bmp) and Sonic Hedgehog (Shh) pathways. In this review, we highlight the function of these pathways in the growth of the cerebellum and the formation of MB. A better understanding of the developmental origins of these tumors will have significant implications for enhancing the treatment of this important childhood cancer.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest related to this work.
Figures


References
-
- Bailey P, Cushing H. A Classification of the Tumors of the Glioma Group on a Histogenetic Basis With a Correlated Study of Prognosis. Lippincott; 1926.
-
- Palay SL, Chan-Palay S. The Cerebellar Cortex: cytology and organization. Springer-Verlag; 1974.
-
- Boyden ES, et al. Cerebellum-dependent learning: The Role of Multiple Plasticity Mechanisms. Annu Rev Neurosci. 2004;27:581–609. - PubMed
-
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Current opinion in neurobiology. 2005;15:667–674. - PubMed
-
- du Lac S, et al. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;18:409–441. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

