Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome
- PMID: 21317170
- PMCID: PMC3112364
- DOI: 10.1136/gut.2010.218271
Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome
Abstract
Background and aims: Teduglutide, a GLP-2 analogue, may restore intestinal structural and functional integrity by promoting repair and growth of the mucosa and reducing gastric emptying and secretion, thereby increasing fluid and nutrient absorption in patients with short bowel syndrome (SBS). This 24-week placebo-controlled study evaluated the ability of teduglutide to reduce parenteral support in patients with SBS with intestinal failure.
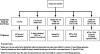
Methods: In 83 patients randomised to receive subcutaneous teduglutide 0.10 mg/kg/day (n = 32), 0.05 mg/kg/day (n = 35) or placebo (n = 16) once daily, parenteral fluids were reduced at 4-week intervals if intestinal fluid absorption (48 h urine volumes) increased ≥ 10%. Responders were subjects who demonstrated reductions of ≥ 20% in parenteral volumes from baseline at weeks 20 and 24. The primary efficacy end point, a graded response score (GRS), took into account higher levels and earlier onset of response, leading to longer duration of response. The intensity of the response was defined as a reduction from baseline in parenteral volume (from 20% to 100%), and the duration of the response was considered the response at weeks 16, 20 and 24. The results were tested according to a step-down procedure starting with the 0.10 mg/kg/day dose.
Results: Using the GRS criteria, teduglutide in a dose of 0.10 mg/kg/day did not have a statistically significant effect compared with placebo (8/32 vs 1/16, p=0.16), while teduglutide in a dose of 0.05 mg/kg/day had a significant effect (16/35, p = 0.007). Since parenteral volume reductions were equal (353 ± 475 and 354 ± 334 ml/day), the trend towards higher baseline parenteral volume (1816 ± 1008 vs 1374 ± 639 ml/day, p=0.11) in the 0.10 mg/kg/day group compared with the 0.05 mg/kg/day group may have accounted for this discrepancy. Three teduglutide-treated patients were completely weaned off parenteral support. Serious adverse events were distributed similarly between active treatment groups and placebo. Villus height, plasma citrulline concentration and lean body mass were significantly increased with teduglutide compared with placebo.
Conclusions: Teduglutide was safe, well tolerated, intestinotrophic and suggested pro-absorptive effects facilitating reductions in parenteral support in patients with SBS with intestinal failure. ClinicalTrials.gov number NCT00172185.
Conflict of interest statement
Figures


References
-
- Messing B, Pigot F, Rongier M, et al. Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 1991;100:1502–8 - PubMed
-
- Fleming CR, Remington M. Intestinal failure. In: Hill GL, ed. Nutrition and the Surgical Patient. New York: Churchill Livingstone, 1981:219–35
-
- O'Keefe SJ, Buchman AL, Fishbein TM, et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006;4:6–10 - PubMed
-
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111–34 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
