Development of an artificial cell, from self-organization to computation and self-reproduction
- PMID: 21317359
- PMCID: PMC3048108
- DOI: 10.1073/pnas.1017075108
Development of an artificial cell, from self-organization to computation and self-reproduction
Abstract
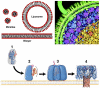
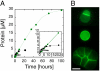
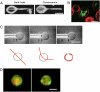
This article describes the state and the development of an artificial cell project. We discuss the experimental constraints to synthesize the most elementary cell-sized compartment that can self-reproduce using synthetic genetic information. The original idea was to program a phospholipid vesicle with DNA. Based on this idea, it was shown that in vitro gene expression could be carried out inside cell-sized synthetic vesicles. It was also shown that a couple of genes could be expressed for a few days inside the vesicles once the exchanges of nutrients with the outside environment were adequately introduced. The development of a cell-free transcription/translation toolbox allows the expression of a large number of genes with multiple transcription factors. As a result, the development of a synthetic DNA program is becoming one of the main hurdles. We discuss the various possibilities to enrich and to replicate this program. Defining a program for self-reproduction remains a difficult question as nongenetic processes, such as molecular self-organization, play an essential and complementary role. The synthesis of a stable compartment with an active interface, one of the critical bottlenecks in the synthesis of artificial cell, depends on the properties of phospholipid membranes. The problem of a self-replicating artificial cell is a long-lasting goal that might imply evolution experiments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schrödinger E. What Is Life? The Physical Aspect of the Living Cell. Cambridge, England: University Press; 1945. p. viii.
-
- Crick F. Life Itself: Its Origin and Nature. New York: Simon & Schuster; 1981.
-
- Lane N. Power, Sex, Suicide: Mitochondria and the Meaning Of Life. Oxford, New York: Oxford Univ Press; 2005. p. xiii.
-
- Von Neumann J. The general and logical theory of automata. In: Jeffress LA, editor. Cerebral Mechanisms in Behavior: The Hixon Symposium. New York: Wiley; 1951.
-
- Kauffman SA. At Home in the Universe: The Search for Laws of Self-Organization and Complexity. New York: Oxford Univ Press; 1995. p. viii.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

