A population-based assessment of specialty physician involvement in cancer clinical trials
- PMID: 21317382
- PMCID: PMC3107589
- DOI: 10.1093/jnci/djq549
A population-based assessment of specialty physician involvement in cancer clinical trials
Abstract
Background: Clinical trials are critical for evaluating new cancer therapies, but few adult patients participate in them. Physicians have an important role in facilitating patient participation in clinical trials. We examined the characteristics of specialty physicians who participate in clinical trials by enrolling or referring patients, the types of trials in which they participate, and factors associated with physicians who report greater involvement in clinical trials.
Methods: We analyzed data from the Cancer Care Outcomes Research and Surveillance Consortium. The study included 1533 specialty physicians who cared for colorectal and lung cancer patients (496 medical oncologists, 228 radiation oncologists, and 809 surgeons) and completed a survey conducted during 2005-2006 (response rate = 61.0%). Descriptive statistics were used to characterize physicians' personal and practice characteristics, and regression models were used to examine associations between these characteristics and physician participation in clinical trials. All statistical tests were two-sided.
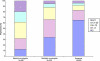
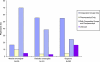
Results: A total of 87.8% of medical oncologists, 66.1% of radiation oncologists, and 35.0% of surgeons reported referring or enrolling one or more patients in clinical trials during the previous 12 months. The mean number of patients referred or enrolled by these physicians was 17.2 (95% confidence interval [CI] = 15.5 to 18.9) for medical oncologists, 9.5 (95% CI = 7.7 to 11.3) for radiation oncologists, and 12.2 (95% CI = 9.8 to 14.6) for surgeons (P < .001). Specialty type, involvement in teaching, and affiliation with a Community Clinical Oncology Program (CCOP) and/or a National Cancer Institute-designated cancer center were associated with physician trial participation and enrolling more patients (all Ps < .05). Two-thirds of physicians with a CCOP or National Cancer Institute-designated cancer center affiliation reported participating in trials.
Conclusions: Features of specialty physicians' practice environments are associated with their trial participation, but many physicians at CCOPs and cancer centers do not participate.
Figures
Comment in
-
Accrual to clinical trials: let's look at the physicians.J Natl Cancer Inst. 2011 Mar 2;103(5):357-8. doi: 10.1093/jnci/djr018. Epub 2011 Feb 11. J Natl Cancer Inst. 2011. PMID: 21317381 No abstract available.
References
-
- National Cancer Institute. NCI Features: Clinical Trials and You. http://www.cancer.gov/clinicaltrials. Accessed December 22, 2010.
-
- Lara PN, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. - PubMed
-
- Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. - PubMed
-
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomized controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. - PubMed
-
- Pinto HA, McCaskill-Stevens W, Wolfe P, Marcus AC. Physician perspectives on increasing minorities in cancer clinical trials: an Eastern Cooperative Oncology Group (ECOG) initiative. Ann Epidemiol. 2000;10(8 suppl):S78–S84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical