Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol's effects
- PMID: 21317387
- PMCID: PMC3271805
- DOI: 10.4049/jimmunol.1003037
Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol's effects
Abstract
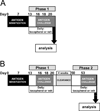
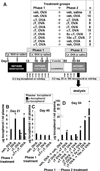
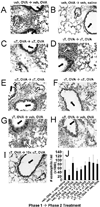
We have reported that supplemental doses of the α- and γ-tocopherol isoforms of vitamin E decrease and increase, respectively, allergic lung inflammation. We have now assessed whether these effects of tocopherols are reversible. For these studies, mice were treated with Ag and supplemental tocopherols in a first phase of treatment followed by a 4-wk clearance phase, and then the mice received a second phase of Ag and tocopherol treatments. The proinflammatory effects of supplemental levels of γ-tocopherol in phase 1 were only partially reversed by supplemental α-tocopherol in phase 2, but were completely reversed by raising α-tocopherol levels 10-fold in phase 2. When γ-tocopherol levels were increased 10-fold (highly elevated tocopherol) so that the lung tissue γ-tocopherol levels were equal to the lung tissue levels of supplemental α-tocopherol, γ-tocopherol reduced leukocyte numbers in the lung lavage fluid. In contrast to the lung lavage fluid, highly elevated levels of γ-tocopherol increased inflammation in the lung tissue. These regulatory effects of highly elevated tocopherols on tissue inflammation and lung lavage fluid were reversible in a second phase of Ag challenge without tocopherols. In summary, the proinflammatory effects of supplemental γ-tocopherol on lung inflammation were partially reversed by supplemental levels of α-tocopherol but were completely reversed by highly elevated levels of α-tocopherol. Also, highly elevated levels of γ-tocopherol were inhibitory and reversible in lung lavage but, importantly, were proinflammatory in lung tissue sections. These results have implications for future studies with tocopherols and provide a new context in which to review vitamin E studies in the literature.
Figures

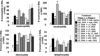







References
-
- Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem. 2004;11:1113–1133. - PubMed
-
- Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. - PubMed
-
- Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev. 2006;64:295–299. - PubMed
-
- Desrumaux C, Deckert V, Athias A, Masson D, Lizard G, Palleau V, Gambert P, Lagrost L. Plasma phospholipid transfer protein prevents vascular endothelium dysfunction by delivering alpha-tocopherol to endothelial cells. FASEB J. 1999;13:883–892. - PubMed
-
- Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

