Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE)
- PMID: 21317434
- PMCID: PMC3070274
- DOI: 10.1136/ard.2010.139261
Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE)
Erratum in
- Ann Rheum Dis. 2011 Jul;70(7):1349
Abstract
Objectives: Patients with advanced ankylosing spondylitis (AS) experience disability because of reduced spinal mobility and pulmonary function impairment. This placebo-controlled study evaluated the effect of etanercept (ETN) in patients with advanced AS.
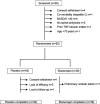
Methods: A multicentre randomised double-blind placebo-controlled trial of 12 weeks' duration was performed. Patients had definite (modified New York criteria), active (Bath AS Disease Activity Index (BASDAI) ≥40), severe (radiological intervertebral bridges) AS refractory to non-steroidal anti-inflammatory drugs and were antitumour necrosis factor naive. They were treated with ETN 50 mg once weekly or identical placebo (PBO).
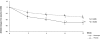
Results: Of the 95 patients screened, 82 were randomised to receive ETN (n=39) or PBO (n=43). At baseline the disease was active (mean BASDAI 61.0±13.4, C reactive protein (CRP) 20.7±25.5 mg/l) and severe (mean Bath AS Metrology Index (BASMI) 5.7±1.3, mSASSS 36.5±20.5); forced pulmonary vital capacity (FVC) was 3.3±0.7 l. Improvement in BASDAI (normalised net incremental area under the curve between baseline and week 12, primary end point) was significantly greater in the ETN group than in the PBO group (-19.8±16.5 vs -11.0±16.4, p=0.019). Moreover, at week 12, ETN gave better results than PBO for the BASDAI (-26.4±19.7 vs -14.4±19.7; p=0.008), total back pain (-29.2±24.0 vs -14.9±24.0; p=0.010), BASFI (-21.7±17.6 vs -10.1±17.6; p=0.004), BASMI (-0.6±0.6 vs -0.2±0.6; p=0.011), CRP level (-15.7±14.2 vs -1.3±14.2; p<0.001) and FVC (+160±280 ml vs -20±280 ml; p=0.006).
Conclusions: ETN has short-term efficacy for patients with advanced AS, as was previously reported for less advanced disease. The efficacy is observed for the main symptoms (pain) and on markers of inflammation (CRP), as well as disease severity in terms of spinal mobility and pulmonary function.
Trial registration: ClinicalTrials.gov NCT00420238.
Conflict of interest statement
Figures
References
-
- Dougados M, Landewé R. Spondyloarthritides: pathogenesis, clinical aspect and diagnosis. In: Bijlsma J, ed. EULAR Compendium on Rheumatic Diseases. London: BMJ Publishing Group; 2009:92–116
-
- Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl 2006;78:4–11 - PubMed
-
- Sampaio-Barros PD, Cerqueira EM, Rezende SM, et al. Pulmonary involvement in ankylosing spondylitis. Clin Rheumatol 2007;26:225–30 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous