Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells
- PMID: 21317533
- PMCID: PMC3049370
- DOI: 10.1172/JCI44876
Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells
Abstract
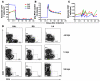
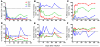
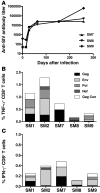
SIV infection of natural host species such as sooty mangabeys results in high viral replication without clinical signs of simian AIDS. Studying such infections is useful for identifying immunologic parameters that lead to AIDS in HIV-infected patients. Here we have demonstrated that acute, SIV-induced CD4(+) T cell depletion in sooty mangabeys does not result in immune dysfunction and progression to simian AIDS and that a population of CD3(+)CD4(-)CD8(-) T cells (double-negative T cells) partially compensates for CD4(+) T cell function in these animals. Passaging plasma from an SIV-infected sooty mangabey with very few CD4(+) T cells to SIV-negative animals resulted in rapid loss of CD4(+) T cells. Nonetheless, all sooty mangabeys generated SIV-specific antibody and T cell responses and maintained normal levels of plasma lipopolysaccharide. Moreover, all CD4-low sooty mangabeys elicited a de novo immune response following influenza vaccination. Such preserved immune responses as well as the low levels of immune activation observed in these animals were associated with the presence of double-negative T cells capable of producing Th1, Th2, and Th17 cytokines. These studies indicate that SIV-infected sooty mangabeys do not appear to rely entirely on CD4(+) T cells to maintain immunity and identify double-negative T cells as a potential subset of cells capable of performing CD4(+) T cell-like helper functions upon SIV-induced CD4(+) T cell depletion in this species.
Figures
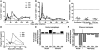
Similar articles
-
Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys.J Virol. 2006 Mar;80(6):2771-83. doi: 10.1128/JVI.80.6.2771-2783.2006. J Virol. 2006. PMID: 16501086 Free PMC article.
-
Multifunctional double-negative T cells in sooty mangabeys mediate T-helper functions irrespective of SIV infection.PLoS Pathog. 2013;9(6):e1003441. doi: 10.1371/journal.ppat.1003441. Epub 2013 Jun 27. PLoS Pathog. 2013. PMID: 23825945 Free PMC article.
-
Paucity of CD4+ natural killer T (NKT) lymphocytes in sooty mangabeys is associated with lack of NKT cell depletion after SIV infection.PLoS One. 2010 Mar 24;5(3):e9787. doi: 10.1371/journal.pone.0009787. PLoS One. 2010. PMID: 20352088 Free PMC article.
-
Double-negative T cells during HIV/SIV infections: potential pinch hitters in the T-cell lineup.Curr Opin HIV AIDS. 2012 Mar;7(2):164-71. doi: 10.1097/COH.0b013e3283504a66. Curr Opin HIV AIDS. 2012. PMID: 22241163 Free PMC article. Review.
-
Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS?J Med Primatol. 2005 Oct;34(5-6):243-52. doi: 10.1111/j.1600-0684.2005.00122.x. J Med Primatol. 2005. PMID: 16128919 Review.
Cited by
-
Prolonged experimental CD4+ T-cell depletion does not cause disease progression in SIV-infected African green monkeys.Nat Commun. 2023 Feb 22;14(1):979. doi: 10.1038/s41467-023-36379-2. Nat Commun. 2023. PMID: 36813761 Free PMC article.
-
Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory.Front Immunol. 2023 Jan 12;13:1060985. doi: 10.3389/fimmu.2022.1060985. eCollection 2022. Front Immunol. 2023. PMID: 36713371 Free PMC article. Review.
-
Elevated Foxp3+ double-negative T cells are associated with disease progression during HIV infection.Front Immunol. 2022 Jul 28;13:947647. doi: 10.3389/fimmu.2022.947647. eCollection 2022. Front Immunol. 2022. PMID: 35967422 Free PMC article.
-
Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors.J Virol. 2015 Oct;89(20):10136-44. doi: 10.1128/JVI.00710-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202248 Free PMC article.
-
Tropism of human pegivirus (formerly known as GB virus C/hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection.J Gen Virol. 2015 Jul;96(Pt 7):1521-32. doi: 10.1099/vir.0.000086. Epub 2015 Feb 9. J Gen Virol. 2015. PMID: 25667328 Free PMC article. Review.
References
-
- Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434(7037):1148–1152. - PubMed
-
- Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

