Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers
- PMID: 21317535
- PMCID: PMC3049400
- DOI: 10.1172/JCI44111
Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers
Abstract
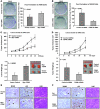
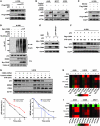
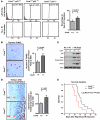
The mammalian constitutive photomorphogenesis 9 (COP9) signalosome (CSN), a protein complex involved in embryonic development, is implicated in cell cycle regulation and the DNA damage response. Its role in tumor development, however, remains unclear. Here, we have shown that the COP9 subunit 6 (CSN6) gene is amplified in human breast cancer specimens, and the CSN6 protein is upregulated in human breast and thyroid tumors. CSN6 expression positively correlated with expression of murine double minute 2 (MDM2), a potent negative regulator of the p53 tumor suppressor. Expression of CSN6 appeared to prevent MDM2 autoubiquitination at lysine 364, resulting in stabilization of MDM2 and degradation of p53. Mice in which Csn6 was deleted died early in embryogenesis (E7.5). Embryos lacking both Csn6 and p53 survived to later in embryonic development (E10.5), which suggests that loss of p53 could partially rescue the effect of loss of Csn6. Mice heterozygous for Csn6 were sensitized to γ-irradiation-induced, p53-dependent apoptosis in both the thymus and the developing CNS. These mice were also less susceptible than wild-type mice to γ-irradiation-induced tumorigenesis. These results suggest that loss of CSN6 enhances p53-mediated tumor suppression in vivo and that CSN6 plays an important role in regulating DNA damage-associated apoptosis and tumorigenesis through control of the MDM2-p53 signaling pathway.
Figures





Similar articles
-
Roles of COP9 signalosome in cancer.Cell Cycle. 2011 Sep 15;10(18):3057-66. doi: 10.4161/cc.10.18.17320. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21876386 Free PMC article. Review.
-
HER2-Akt signaling in regulating COP9 signalsome subunit 6 and p53.Cell Cycle. 2012 Nov 15;11(22):4181-90. doi: 10.4161/cc.22413. Epub 2012 Oct 24. Cell Cycle. 2012. PMID: 23095642 Free PMC article.
-
CDK inhibitor p57 (Kip2) is negatively regulated by COP9 signalosome subunit 6.Cell Cycle. 2012 Dec 15;11(24):4633-41. doi: 10.4161/cc.22887. Epub 2012 Nov 27. Cell Cycle. 2012. PMID: 23187808 Free PMC article.
-
CSN6 promotes the cell migration of breast cancer cells by positively regulating Snail1 stability.Int J Med Sci. 2020 Oct 1;17(17):2809-2818. doi: 10.7150/ijms.50206. eCollection 2020. Int J Med Sci. 2020. PMID: 33162808 Free PMC article.
-
The Emerging Role of CSN6 in Biological Behavior and Cancer Progress.Anticancer Agents Med Chem. 2019;19(10):1198-1204. doi: 10.2174/1871520619666190408142131. Anticancer Agents Med Chem. 2019. PMID: 30961513 Review.
Cited by
-
From photomorphogenesis to cancer: a CSN journey.Cell Cycle. 2013 Jan 15;12(2):205-6. doi: 10.4161/cc.23422. Epub 2012 Jan 15. Cell Cycle. 2013. PMID: 23287466 Free PMC article. No abstract available.
-
The COP9 signalosome subunit 6 (CSN6): a potential oncogene.Cell Div. 2013 Nov 28;8(1):14. doi: 10.1186/1747-1028-8-14. Cell Div. 2013. PMID: 24286178 Free PMC article.
-
The Involvement of Ubiquitination Machinery in Cell Cycle Regulation and Cancer Progression.Int J Mol Sci. 2021 May 27;22(11):5754. doi: 10.3390/ijms22115754. Int J Mol Sci. 2021. PMID: 34072267 Free PMC article. Review.
-
COP9 subunits 4 and 5 target soluble guanylyl cyclase α1 and p53 in prostate cancer cells.Mol Endocrinol. 2014 Jun;28(6):834-45. doi: 10.1210/me.2014-1017. Epub 2014 Apr 11. Mol Endocrinol. 2014. PMID: 24725084 Free PMC article.
-
DCAF1 controls T-cell function via p53-dependent and -independent mechanisms.Nat Commun. 2016 Jan 5;7:10307. doi: 10.1038/ncomms10307. Nat Commun. 2016. PMID: 26728942 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

