IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway
- PMID: 21317537
- PMCID: PMC3049373
- DOI: 10.1172/JCI43475
IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway
Abstract
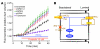
Fluid and HCO(3)(-) secretion are fundamental functions of epithelia and determine bodily fluid volume and ionic composition, among other things. Secretion of ductal fluid and HCO(3)(-) in secretory glands is fueled by Na(+)/HCO(3)(-) cotransport mediated by basolateral solute carrier family 4 member 4 (NBCe1-B) and by Cl(-)/HCO(3)(-) exchange mediated by luminal solute carrier family 26, member 6 (Slc26a6) and CFTR. However, the mechanisms governing ductal secretion are not known. Here, we have shown that pancreatic ductal secretion in mice is suppressed by silencing of the NBCe1-B/CFTR activator inositol-1,4,5-trisphosphate (IP(3)) receptor-binding protein released with IP(3) (IRBIT) and by inhibition of protein phosphatase 1 (PP1). In contrast, silencing the with-no-lysine (WNK) kinases and Ste20-related proline/alanine-rich kinase (SPAK) increased secretion. Molecular analysis revealed that the WNK kinases acted as scaffolds to recruit SPAK, which phosphorylated CFTR and NBCe1-B, reducing their cell surface expression. IRBIT opposed the effects of WNKs and SPAK by recruiting PP1 to the complex to dephosphorylate CFTR and NBCe1-B, restoring their cell surface expression, in addition to stimulating their activities. Silencing of SPAK and IRBIT in the same ducts rescued ductal secretion due to silencing of IRBIT alone. These findings stress the pivotal role of IRBIT in epithelial fluid and HCO(3)(-) secretion and provide a molecular mechanism by which IRBIT coordinates these processes. They also have implications for WNK/SPAK kinase-regulated processes involved in systemic fluid homeostasis, hypertension, and cystic fibrosis.
Figures




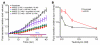

Similar articles
-
Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3- cotransporters family.Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4105-10. doi: 10.1073/pnas.1221410110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431199 Free PMC article.
-
IRBIT coordinates epithelial fluid and HCO3- secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct.J Clin Invest. 2009 Jan;119(1):193-202. doi: 10.1172/JCI36983. Epub 2008 Dec 1. J Clin Invest. 2009. PMID: 19033647 Free PMC article.
-
Modulation of Cl- signaling and ion transport by recruitment of kinases and phosphatases mediated by the regulatory protein IRBIT.Sci Signal. 2018 Oct 30;11(554):eaat5018. doi: 10.1126/scisignal.aat5018. Sci Signal. 2018. PMID: 30377224 Free PMC article.
-
The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport.Physiology (Bethesda). 2012 Oct;27(5):291-9. doi: 10.1152/physiol.00028.2012. Physiology (Bethesda). 2012. PMID: 23026752 Free PMC article. Review.
-
IRBIT: a regulator of ion channels and ion transporters.Biochim Biophys Acta. 2014 Oct;1843(10):2195-204. doi: 10.1016/j.bbamcr.2014.01.031. Epub 2014 Feb 8. Biochim Biophys Acta. 2014. PMID: 24518248 Review.
Cited by
-
Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport.Physiol Rev. 2012 Oct;92(4):1577-617. doi: 10.1152/physrev.00009.2012. Physiol Rev. 2012. PMID: 23073627 Free PMC article. Review.
-
Chaperone stress 70 protein (STCH) binds and regulates two acid/base transporters NBCe1-B and NHE1.J Biol Chem. 2013 Mar 1;288(9):6295-305. doi: 10.1074/jbc.M112.392001. Epub 2013 Jan 9. J Biol Chem. 2013. PMID: 23303189 Free PMC article.
-
Multiple Regulatory Signals and Components in the Modulation of Bicarbonate Transporters.Pharmaceutics. 2024 Jan 5;16(1):78. doi: 10.3390/pharmaceutics16010078. Pharmaceutics. 2024. PMID: 38258089 Free PMC article. Review.
-
Proximal renal tubular acidosis mediated by mutations in NBCe1-A: unraveling the transporter's structure-functional properties.Front Physiol. 2013 Dec 19;4:350. doi: 10.3389/fphys.2013.00350. Front Physiol. 2013. PMID: 24391589 Free PMC article. Review.
-
K+/Cl- cotransporter 2 (KCC2) and Na+/ cotransporter 1 (NBCe1) interaction modulates profile of KCC2 phosphorylation.Front Cell Neurosci. 2023 Oct 10;17:1253424. doi: 10.3389/fncel.2023.1253424. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37881493 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

