ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma
- PMID: 21317715
- PMCID: PMC6880747
- DOI: 10.1097/PAS.0b013e318206b67b
ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma
Abstract
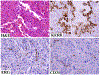
ERG, an ETS family transcription factor, is known to be expressed in endothelial cells, and oncogenic ERG gene fusions occur in subsets of prostatic carcinoma, acute myeloid leukemia, and Ewing sarcoma. In this study, we immunohistochemically investigated nuclear ERG expression using a new monoclonal antibody, CPDR ERG-MAb, that is highly specific for detecting ERG protein and ERG-expressing prostate carcinomas. A broad range of vascular endothelial (n = 250), other mesenchymal (n = 973), and epithelial tumors (n = 657) was examined to determine the use of ERG immunohistochemistry in surgical pathology. Only immunostains with ERG-positive normal endothelia (internal control) were considered valid, and only nuclear staining was considered to be positive. In adult tissues, ERG was restricted to endothelial cells and to a subset of bone marrow precursors, but early fetal mesenchyme and subpopulations of fetal cartilage were also positive. In vascular tumors, ERG was expressed in endothelia of all hemangiomas and lymphangiomas, and typically extensively expressed in 96 of 100 angiosarcomas, 42 of 43 epithelioid hemangioendotheliomas, and all 26 Kaposi sarcomas. Among nonvascular mesenchymal tumors, only blastic extramedullary myeloid tumors (7 of 10) and rare Ewing sarcomas (2 of 29) were positive. Among epithelial tumors, 30 of 66 prostatic adenocarcinomas showed focal-to-extensive ERG positivity, with no immunoreactivity in the normal prostate. Other carcinomas and epithelial tumors (n = 643) were ERG negative, with the exception of 1 of 42 large cell undifferentiated pulmonary carcinomas and 1 of 27 mesotheliomas, each of which showed focal nuclear ERG positivity. On the basis of the above observations, ERG is a highly specific new marker for benign and malignant vascular tumors. Among epithelial tumors, ERG shows a great promise as a marker to identify prostatic carcinoma in both primary and metastatic settings.
Figures





References
-
- Baltzinger M, Mager-Heckel AM, Remy P. XI erg: Expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev Dyn 1999;216:420–433. - PubMed
-
- de Young BR, Wick MR, Fitzgibbon JF, et al. CD31: An immunospecific marker for endothelial differentiation in human neoplasms. Appl Immunohistochem 1993;1:97–100.
-
- Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001;25:1061–1066. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

