Plant polyphenols and their anti-cariogenic properties: a review
- PMID: 21317840
- PMCID: PMC6259836
- DOI: 10.3390/molecules16021486
Plant polyphenols and their anti-cariogenic properties: a review
Abstract
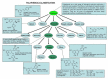
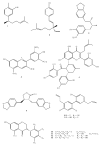
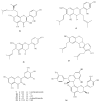
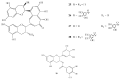
Polyphenols constitute one of the most common groups of substances in plants. Polyphenolic compounds have been reported to have a wide range of biological activities, many of which are related to their conventional antioxidant action; however, increasing scientific knowledge has highlighted their potential activity in preventing oral disease, including the prevention of tooth decay. The aim of this review is to show the emerging findings on the anti-cariogenic properties of polyphenols, which have been obtained from several in vitro studies investigating the effects of these bioactive molecules against Streptococcus mutans, as well as in vivo studies. The analysis of the literature supports the anti-bacterial role of polyphenols on cariogenic streptococci, suggesting (1) a direct effect against S. mutans; (2) an interaction with microbial membrane proteins inhibiting the adherence of bacterial cells to the tooth surface; and (3) the inhibition of glucosyl transferase and amylase. However, more studies, particularly in vivo and in situ, are necessary to establish conclusive evidence for the effectiveness and the clinical applications of these compounds in the prevention of dental caries. It is essential to better determine the nature and distribution of these compounds in our diet and to identify which of the hundreds of existing polyphenols are likely to provide the greatest effects.
Figures
References
-
- Arakawa H., Maeda M., Okubo S., Shimamura T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004;27:277–281. - PubMed
-
- Banas J.A., Vickerman M.M. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003;14:89–99. - PubMed
-
- Bernaert H., Allegaert L. Topical Skin Cosmetics Comprising a Cocoa Polyphenol Extract Combination with SUS-Rich Fat. 2009/0233518 A1. Fat. U.S. Patent. 2009 October ;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

