Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response
- PMID: 21317875
- PMCID: PMC3049214
- DOI: 10.1038/emboj.2011.18
Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response
Abstract
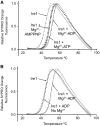
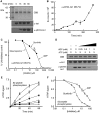
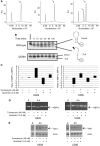
Ire1 (Ern1) is an unusual transmembrane protein kinase essential for the endoplasmic reticulum (ER) unfolded protein response (UPR). Activation of Ire1 by association of its N-terminal ER luminal domains promotes autophosphorylation by its cytoplasmic kinase domain, leading to activation of the C-terminal ribonuclease domain, which splices Xbp1 mRNA generating an active Xbp1s transcriptional activator. We have determined the crystal structure of the cytoplasmic portion of dephosphorylated human Ire1α bound to ADP, revealing the 'phosphoryl-transfer' competent dimeric face-to-face complex, which precedes and is distinct from the back-to-back RNase 'active' conformation described for yeast Ire1. We show that the Xbp1-specific ribonuclease activity depends on autophosphorylation, and that ATP-competitive inhibitors staurosporin and sunitinib, which inhibit autophosphorylation in vitro, also inhibit Xbp1 splicing in vivo. Furthermore, we demonstrate that activated Ire1α is a competent protein kinase, able to phosphorylate a heterologous peptide substrate. These studies identify human Ire1α as a target for development of ATP-competitive inhibitors that will modulate the UPR in human cells, which has particular relevance for myeloma and other secretory malignancies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

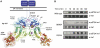
References
-
- Adams PD, Gopal K, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Pai RK, Read RJ, Romo TD, Sacchettini JC, Sauter NK, Storoni LC, Terwilliger TC (2004) Recent developments in the PHENIX software for automated crystallographic structure determination. J Synchrotron Radiat 11: 53–55 - PubMed
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332 - PubMed
-
- Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, Munshi N, Horner J, Ivanova EV, Protopopov A, Anderson KC, Tonon G, DePinho RA (2007) The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11: 349–360 - PMC - PubMed
-
- CCP4 (1994) Programs for protein crystallography. Acta Crystallogr D 50: 760–763 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

