A reservoir of time constants for memory traces in cortical neurons
- PMID: 21317906
- PMCID: PMC3079398
- DOI: 10.1038/nn.2752
A reservoir of time constants for memory traces in cortical neurons
Abstract
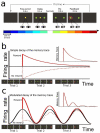
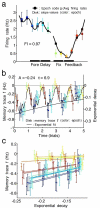
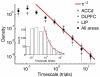
According to reinforcement learning theory of decision making, reward expectation is computed by integrating past rewards with a fixed timescale. In contrast, we found that a wide range of time constants is available across cortical neurons recorded from monkeys performing a competitive game task. By recognizing that reward modulates neural activity multiplicatively, we found that one or two time constants of reward memory can be extracted for each neuron in prefrontal, cingulate and parietal cortex. These timescales ranged from hundreds of milliseconds to tens of seconds, according to a power law distribution, which is consistent across areas and reproduced by a 'reservoir' neural network model. These neuronal memory timescales were weakly, but significantly, correlated with those of monkey's decisions. Our findings suggest a flexible memory system in which neural subpopulations with distinct sets of long or short memory timescales may be selectively deployed according to the task demands.
Figures
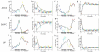
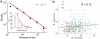
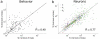

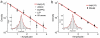
References
-
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008;11(4):389–97. - PubMed
-
- Sutton RS, Barto AG. Reinforcement Learning, An Introduction. MIT Press; Cambridge, MA: 1998.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

