The waking brain: an update
- PMID: 21318261
- PMCID: PMC3134769
- DOI: 10.1007/s00018-011-0631-8
The waking brain: an update
Abstract
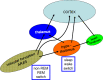
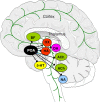
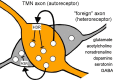
Wakefulness and consciousness depend on perturbation of the cortical soliloquy. Ascending activation of the cerebral cortex is characteristic for both waking and paradoxical (REM) sleep. These evolutionary conserved activating systems build a network in the brainstem, midbrain, and diencephalon that contains the neurotransmitters and neuromodulators glutamate, histamine, acetylcholine, the catecholamines, serotonin, and some neuropeptides orchestrating the different behavioral states. Inhibition of these waking systems by GABAergic neurons allows sleep. Over the past decades, a prominent role became evident for the histaminergic and the orexinergic neurons as a hypothalamic waking center.
Figures


References
-
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. - PubMed
-
- Bremer F. Cerveau isolé et physiologie du sommeil. C R Soc Biol (Paris) 1935;118:1235–1242.
-
- Jouvet M (1993) From amines to sleep—a citation-classic commentary on the role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle by Jouvet, M. Curr Contents Life Sci 8 - PubMed
-
- Moruzzi G (1972) The sleep-waking cycle. Ergebnisse der Physiologie Biologischen Chemie und Experimentellen Pharmakologie 64:1 - PubMed
-
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum Press; 1990.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

