Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: the CHOICE trial
- PMID: 21319147
- PMCID: PMC3136553
- DOI: 10.1002/cncr.25924
Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: the CHOICE trial
Abstract
Background: Colorectal cancer (CRC) screening reduces CRC incidence and mortality but is underused. Effective interventions to increase screening that can be implemented broadly are needed.
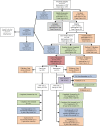
Methods: A controlled trial was conducted to evaluate a patient-level and practice-level intervention to increase the use of recommended CRC screening tests among health plan members. The patient-level intervention was a patient decision aid and included stage-targeted brochures that were mailed to health plan members. Intervention practices received academic detailing to prepare practices to facilitate CRC testing once patients were activated by the decision aid. We used patient surveys and claims data to assess CRC test completion.
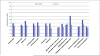
Results: Among 443 active participants, 75.8% were ages 52 to 59 years, 80.9% were white, 62.1% were women, and 46.4% had college degrees or greater education. Among 380 active participants with known screening status at 12 months based on survey results, 39% in the intervention group reported receiving CRC screening compared with 32.2% in the usual care group (unadjusted odds ratio [OR], 1.34; 95% confidence interval; [CI], 0.88-2.05; P = .17). After adjusting for baseline differences and accounting for clustering, the effect was somewhat larger (OR, 1.64; 95% CI, 0.98-2.73; P = .06). Claims analysis produced similar effects for active participants. The intervention was more effective in those who had incomes >$50,000 (OR, 2.16; 95% CI, 1.07-4.35) than in those who had lower incomes (OR, 1.25; 95% CI, 0.53-2.94; P = .03 for interaction).
Conclusions: Interventions combining a patient-directed decision aid and practice-directed academic detailing had a modest but statistically nonsignificant effect on CRC screening rates among active participants.
Trial registration: ClinicalTrials.gov NCT00134589.
Copyright © 2011 American Cancer Society.
Figures
References
-
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–58. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):96–104. Available from http://www.annals.org/cgi/reprint/137/2/96.pdf. - PubMed
-
- Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31(1):80–9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1623–30. - PubMed
-
- Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–94. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical