Profiling impaired hepatic endoplasmic reticulum glycosylation as a consequence of ethanol ingestion
- PMID: 21319786
- PMCID: PMC3501204
- DOI: 10.1021/pr101101s
Profiling impaired hepatic endoplasmic reticulum glycosylation as a consequence of ethanol ingestion
Abstract
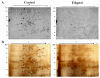
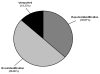
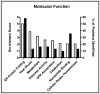
Alcoholic liver disease (ALD) is a prominent cause of morbidity and mortality in the United States. Alterations in protein folding occur in numerous disease states, including ALD. The endoplasmic reticulum (ER) is the primary site of post-translational modifications (PTM) within the cell. Glycosylation, the most abundant PTM, affects protein stability, structure, localization, and activity. Decreases in hepatic glycosylation machinery have been observed in rodent models of ALD, but specific protein targets have not been identified. Utilizing two-dimensional gel electrophoresis and liquid chromatography-tandem mass spectrometry, glycoproteins were identified in hepatic microsomal fractions from control and ethanol-fed mice. This study reports for the first time a global decrease in ER glycosylation. Additionally, the identification of 30 glycoproteins within this fraction elucidates pathway-specific alterations in ALD impaired glycosylation. Among the identified proteins, triacylglycerol hydrolase (TGH) is positively affected by glycosylation, showing increased activity following the addition of sugar moieties. Impaired TGH activity is associated with increased cellular storage of lipids and provides a potential mechanism for the observed pathologies associated with ALD.
Figures



Similar articles
-
Protein carbonylation in a murine model for early alcoholic liver disease.Chem Res Toxicol. 2012 May 21;25(5):1012-21. doi: 10.1021/tx300002q. Epub 2012 May 1. Chem Res Toxicol. 2012. PMID: 22502949 Free PMC article.
-
Proteomic Profiling of Liver and Plasma in Chronic Ethanol Feeding Model of Hepatic Alcohol Dehydrogenase-Deficient Deer Mice.Alcohol Clin Exp Res. 2017 Oct;41(10):1675-1685. doi: 10.1111/acer.13470. Epub 2017 Sep 8. Alcohol Clin Exp Res. 2017. PMID: 28792616 Free PMC article.
-
Soluble Epoxide Hydrolase Hepatic Deficiency Ameliorates Alcohol-Associated Liver Disease.Cell Mol Gastroenterol Hepatol. 2021;11(3):815-830. doi: 10.1016/j.jcmgh.2020.10.002. Epub 2020 Oct 15. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33068774 Free PMC article.
-
Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease.Chem Biol Interact. 2011 Jun 30;192(1-2):107-12. doi: 10.1016/j.cbi.2011.02.021. Epub 2011 Feb 24. Chem Biol Interact. 2011. PMID: 21354120 Free PMC article. Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics.PLoS One. 2012;7(6):e38459. doi: 10.1371/journal.pone.0038459. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701647 Free PMC article.
-
Liver-Specific Deletion of Phosphatase and Tensin Homolog Deleted on Chromosome 10 Significantly Ameliorates Chronic EtOH-Induced Increases in Hepatocellular Damage.PLoS One. 2016 Apr 28;11(4):e0154152. doi: 10.1371/journal.pone.0154152. eCollection 2016. PLoS One. 2016. PMID: 27124661 Free PMC article.
-
Short term feeding of a high fat diet exerts an additive effect on hepatocellular damage and steatosis in liver-specific PTEN knockout mice.PLoS One. 2014 May 12;9(5):e96553. doi: 10.1371/journal.pone.0096553. eCollection 2014. PLoS One. 2014. PMID: 24818992 Free PMC article.
-
Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes.Hepatology. 2012 Aug;56(2):594-604. doi: 10.1002/hep.25702. Epub 2012 Jun 11. Hepatology. 2012. PMID: 22407670 Free PMC article.
-
Proteomic analysis of plasma proteins during fentanyl withdrawal in ovariectomized female rats with and without estradiol.J Neuroendocrinol. 2025 Aug;37(8):e70033. doi: 10.1111/jne.70033. Epub 2025 Apr 20. J Neuroendocrinol. 2025. PMID: 40254411 Free PMC article.
References
-
- Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27(4):367–77. - PubMed
-
- Seitz O. Glycopeptide synthesis and the effects of glycosylation on protein structure and activity. Chembiochem. 2000;1(4):214–46. - PubMed
-
- Blomme B, Van Steenkiste C, Callewaert N, Van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J Hepatol. 2009;50(3):592–603. - PubMed
-
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

